Newsletter
CRSIPR Transgenic Mice
Service to generate transgenic mice using BAC or Random insertion. Applied StemCell has a strong service portfolio for generating transgenic mouse models using its CRISPR, including conditional knockout and large fragment knock-in (up to 20 kb) models. We are also very experienced in generating mouse models using traditional homologous recombination via embryonic stem cells (ESCs).
Our animal specialists can also generate transgenic mouse models using different approaches to meet your specific needs.
(1) Bacterial artificial chromosome (BAC) to insert the complete foreign gene along with the promoter, introns and other required regulatory elements for gene expression
(2) Random microinjection of the foreign gene into the pronucleus of a fertilized mouse egg: Please note that the expression of the transgene and the subsequent transgenic mouse phenotype may vary from line to line due to the nature of random gene insertion.
We recommend generating multiple independent transgenic mouse lines in order to interpret the experimental results.
- Affordable! Low prices and founder guarantee
- F1 breeding for germline transmission
- Novel CRISPR strategy for higher efficiency and success rate
- Animal IP belongs to customers
- Fast turnaround
- Free consultation
- Downstream assay services - available
- We also offer a repository of off-shelf mouse models
Other Available Mouse Generation Services
Products and Services
Technical Details
Generation of transgenic mice by BAC cloning
Step 1: Design and cloning of gene of interest, promoter, and regulatory elements into BAC vector
Step 2: Pronuclear microinjection of vector DNA into mouse embryos
Step 3: Transfer of injected embryo into recipient mothers
Step 4: Genotyping of offspring to identify potential transgenic founders
Step 5: Breeding to F1 or F2 progenies (optional)
Generation of transgenic mice by random insertion
Step 1: Cloning of cDNA, promoter of interest, and other elements
Step 2: Construction of the transgene vector
Step 3: Pronuclear microinjection of vector DNA into mouse embryos
Step 4: Transfer of injected embryo into recipient mothers
Step 5: Genotyping of offspring to identify potential transgenic founders
Step 6: Breeding to F1 or F2 progenies (optional)
Case Studies
Case Studies for Transgenic Mice Generated by Pronuclear Microinjection
Case #1: Knock-in of a cell proliferation inhibitor gene in C57BL6/N mice by microinjection of DNA of interest directly into the pronucleus of a fertilized mouse egg.
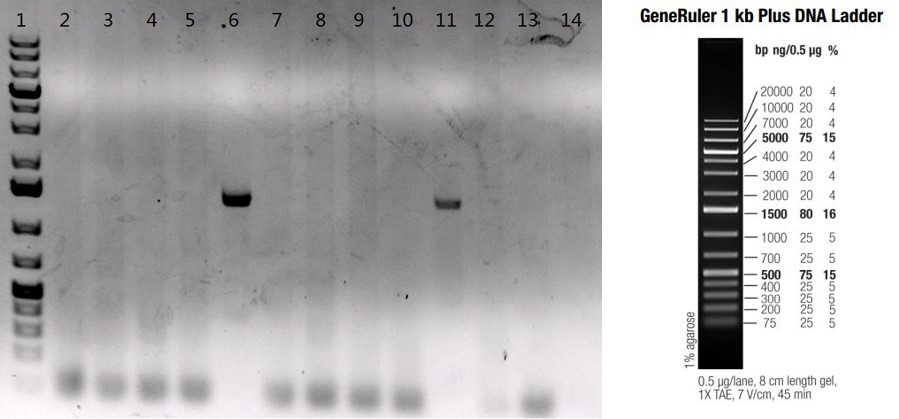
Figure: PCR confirmation of gene knock-in by random insertion into C57BL6/N mice. Twelve pups were born from two rounds of microinjection. Two pups (lane #6, lane #11) were confirmed to carry the inhibitor gene by PCR; lanes #2-13: twelve F0 pups; lane #1: GeneRulerTM 1kb plus DNA ladder; lane #14: B6/N negative control.
Case #2: Knock-in of a NAD/NADH related enzyme gene in C57BL6/N mice by microinjection of DNA of interest directly into the pronucleus of a fertilized mouse egg.
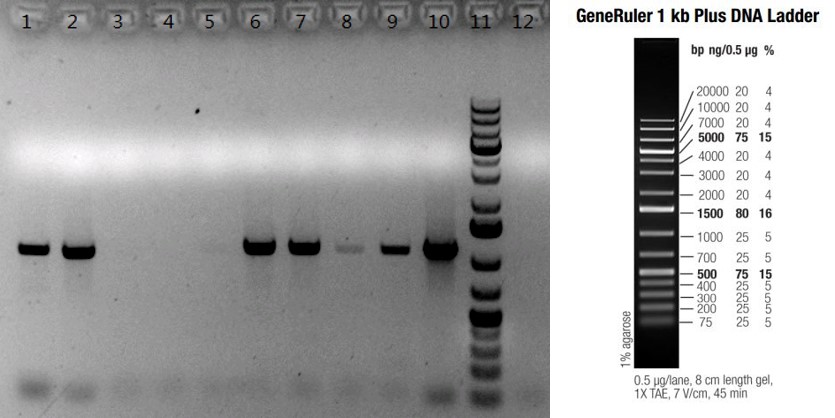
Figure: PCR confirmation of gene knock-in by random insertion in C57BLB6/N mice. Ten pups were born from one microinjection experiment. Seven of them (Lanes #1, 2, 6, 7, 8, 9 and 10) were confirmed to have the enzyme gene knock-in by PCR; lanes #1-10: ten F0 pups; lane #11: GeneRulerTM 1kb plus DNA Ladder; lane #12: B6/N negative control.
Publications
Mouse/ Rat Models: Homologous Recombination Conditional Knockout Mouse
- Geraets, R. D. (2019). Neuronal Ceroid Lipfuscinosis: A Tailored Animal Model of CLN2 Disease and Evaluation of Select Personalized Therapies (Doctoral dissertation, ProQuest Dissertations Publishing).
- Zhao, M., Tao, F., Venkatraman, A., Li, Z., Smith, S. E., Unruh, J., ... & Marshall, H. (2019). N-Cadherin-Expressing Bone and Marrow Stromal Progenitor Cells Maintain Reserve Hematopoietic Stem Cells. Cell reports, 26(3), 652-669.
-
Li, C., Zheng, Z., Ha, P., Chen, X., Jiang, W., Sun, S., ... & Chen, E. C. (2018). Neurexin Superfamily Cell Membrane Receptor Contactin‐Associated Protein Like‐4 (Cntnap4) is Involved in Neural EGFL Like 1 (Nell‐1)‐responsive Osteogenesis. Journal of Bone and Mineral Research https://doi.org/10.1002/jbmr.3524.
-
Geraets, R. D., Langin, L. M., Cain, J. T., Parker, C. M., Beraldi, R., Kovacs, A. D., ... & Pearce, D. A. (2017). A tailored mouse model of CLN2 disease: A nonsense mutant for testing personalized therapies. PloS one, 12(5), e0176526.
-
Miller, J. N., Kovács, A. D., & Pearce, D. A. (2015). The novel Cln1R151Xmouse model of infantile neuronal ceroid lipofuscinosis (INCL) for testing nonsense suppression therapy. Human Molecular Genetics, 24(1), 185–196. http://doi.org/10.1093/hmg/ddu428.
For more journal references, please visit our comprehensive list of citations and reference publications.



