Newsletter
TECHNICAL DETAILS: iPSC-BASED NEUROTOXICITY AND DRUG SCREENING
Drug Screening for Neurological Disorders using iPSC-derived Neuronal Lineage Cells
Using iPSC-differentiated Neural Lineage Cells for Determining Cell Viability in Neurotoxicity Assays
|
A.
|
B.
|
|
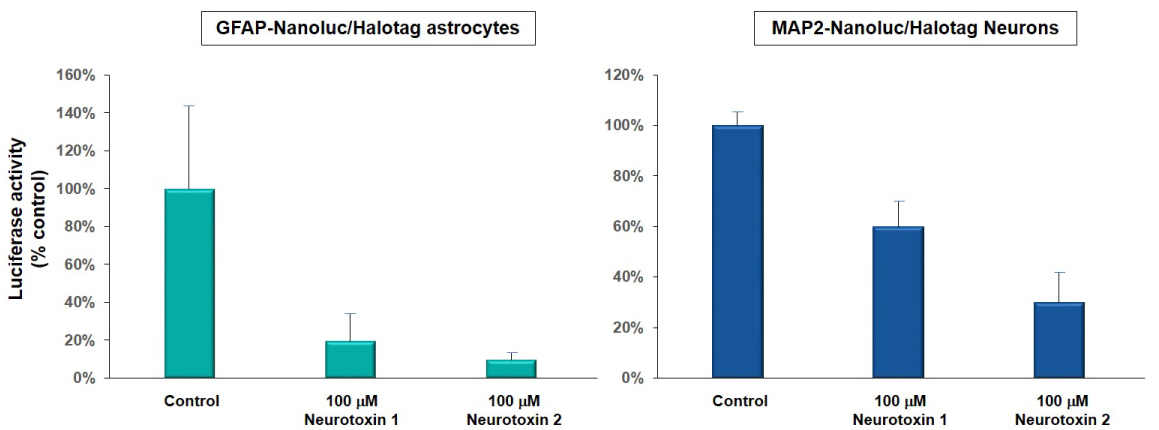
Figure 1A. Luciferase activity (bioluminescence) was used to detect the cell viability of astrocytes and neurons derived from neural reporter iPSC lines (GFAP-Nanoluc/Halotag, ASE-9501; green and MAP2/Nanoluc-Halotag, ASE-9500; blue) when exposed to 100 µM of neurotoxin 1 and 2. Neurotoxin 1 reduced luciferase activity in astrocytes by ~ 80% and in neurons by ~40%. Neurotoxin 2 reduced luciferase activity in astrocytes by ~90% and in neurons by ~70%. Luciferase activity was measured (as % of control; DMSO-treated cells) to determine the extent of cytotoxicity of the compounds. These results were similar to toxicity measured by the MTT assay. |
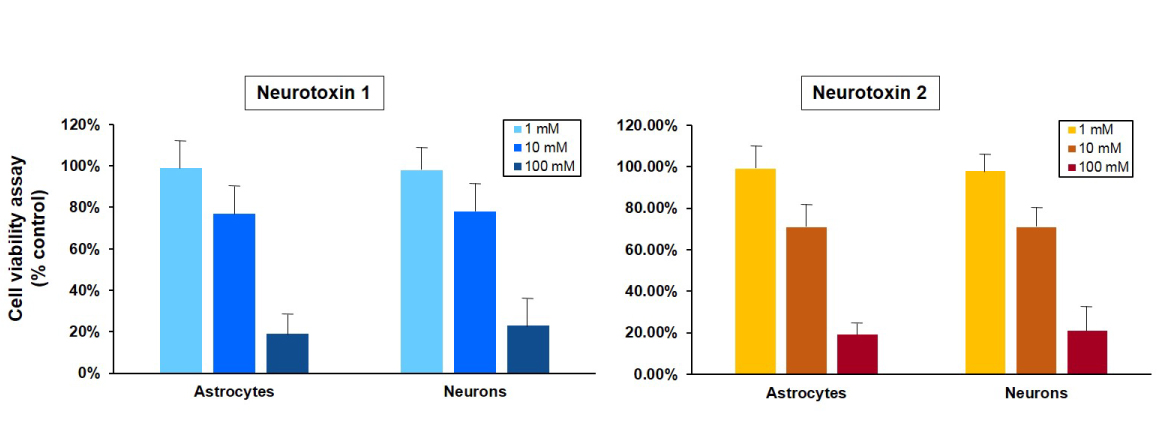
Figure 1B. Cell viability of astrocytes and neurons after exposure to neurotoxins. Astrocytes and neurons derived from a control iPSC line (ASE-9109) were exposed to concentrations of 1, 10 and 100 μM concentrations of two neurotoxins, 1 and 2. Ce ll viability was evaluated using MTT assay (MTT tetrazolium salt) and cell survival was expressed as % of absorbance of viable cells normalized to control (DMSO-treated cells). The 1 µM concentration of both neurotoxins was not significantly cytotoxic in both cell types while there was mild cytotoxicity observed at the 10µM concentrations of the neurotoxins. The 100 µM concentration of both neurotoxins was significantly cytotoxic and resulted in ~80% reduction in cell viability in astrocytes and neurons. The results of this assay were similar to the expression of luciferase in astrocytes and neurons derived from a neuronal-reporter iPSC line (Figure 1A.). |
iPSC-differentiated Neurons Can be Used for Screening Potential Neuroprotective Compounds
iPSCs and derived neurons can be used for testing neuroprotective effects of compoinds using MTT asasy.
Table 1. Drugs that were neuroprotective in iPSC and derived neuronal cells, and used for human clinical trials
|
Neurotransmitter/ MAO Inhibitors: |
Rasagiline, selegiline, nicotine, topiramate, amantadine, zonisamide, taurine |
|
Antioxidant/ Mitochondrial Stabilizers: |
Resveratrol, N-acetyl cysteine, lipoic acid, epigallocatechin gallate, creatine |
|
Anti-inflammatories: |
Rolipram, indomethacin, 7-nitroindazole, 3-aminobenzamide, phenanthridone |
Table 2. Drug that were not neuroprotective in iPSC-based models but were neuroprotective in conventional cell lines and animal models:
|
Neurotransmitter/ MAO Inhibitors: |
Donepezil, caffeine, theophylline, pergolide, apomorphine, riluzole, pramipexole |
|
Antioxidant/ Mitochondrial Stabilizers: |
Ascorbic acid, coenzyme Q10, uric acid, folic acid, ropinirole |
|
Anti-inflammatories: |
Minocycline, estradiol, clioquinol, plicamycin |
Dopaminergic (DA) neurons derived from control iPSC lines were used to screen neurological compounds that have been shown to be neuroprotective in rodent and cell line models (Table 1 and 2). These derived-primary DA neurons were grown in 96-well plates and pretreated with one of each of the selected compounds. The cells were then exposed to either rotenone or MPP+ that are commonly used in dopamine toxicity models and cell viability was determined using the MTT assay. Only 18 out of the compounds (Table 1) were found to be neuroprotective in these iPSC-derived DA neurons, and these 18 compounds have been used in human Parkinson's disease clinical trials to test for neuroprotection. The rest of the compounds (Table 2) have either not been used in clinical trials or were not neuroprotective. This highlights the importance of using iPSC-derived neurons for large-scale screening neurological drugs before evaluating their efficacy in animal and human studies.