Newsletter
GMP TARGATT™ iPSC-iNK Platform
iPSC Derived NK, CAR-T/CAR-NK Cell Generation
As natural killer (NK) cell-based cancer immunotherapy research continues to grow, so has the number of potential chimeric antigen receptor (CAR)-NK therapies. Researchers continue to explore the cytotoxic capabilities of engineered NK cells, but several CAR-NK cell generation problems remain. For example, the CAR-NK cell development process takes several weeks, the patient’s NK cells may be weak and in a damaged state, or cancer cells may be created with random integration. Recent advancements in induced pluripotent stem cell (iPSC) technology have opened the door for the development of CAR-iPSCs that can be further differentiated into NK cells. These cells could potentially fix the current CAR-NK problems the research community is facing.
Applied StemCell (ASC) combined its optimized TARGATTTM gene editing and NK differentiation technologies to establish the TARGATTTM iPSC-iNK Platform. Our expert scientists can insert your specific CAR genetic material into our iPSCs at a safe harbor locus with an efficiency ~10x better than CRISPR and further differentiate your CAR-iPSCs to high-quality NK cells. Leverage our unlimited source of iPSCs and proprietary TARGATTTM technology to safely and efficiently produce the allogenic iNK cells you need to drive your research forward.
ASC Advantages
- >40% gene integration efficiency
- Site-specific knock-in
- NK cells derived from an unlimited source of iPSCs
- iPSC-iNK cell banks with a consistent manufacturing process
- Safe and efficient gene editing protocols
- Transfection by lipofectamine, eliminating viral manufacturing
- We generate truly off-the-shelf allogeneic therapeutic cells
As a CRO/CDMO service provider that continuously works to improve and expand its technology, services, and products, we hope to have our new cGMP TARGATT™ iPSCs available for purchase early next year. For now, we offer you fully characterized cGMP grade iPSCs from CD34+ cord blood. Contact us today to learn more.
Why Applied StemCell?
We understand working with iPSCs remains difficult, and you may encounter several challenges. At ASC our stem cell and gene editing experts are ready to help you every step of the way.
1. Send in your CAR design for review. Our experts can help you enhance your design.
2. Our team can design research and GMP projects in parallel OR generate a matching research grade cell line for preliminary testing.
3. Downstream Assay Services Are Available: cytotoxicity assays, in vivo testing, drug banking, and more - Inquire
Service Overview
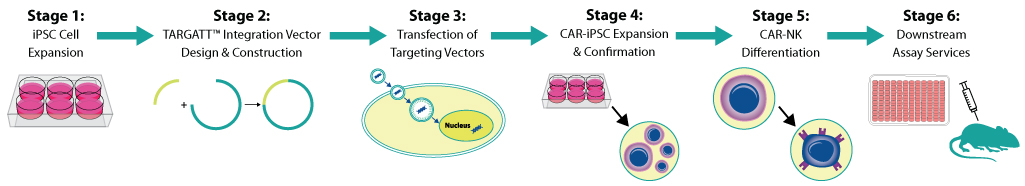
|
Stage 1: iPSC Cell Expansion |
Stage 2: TARGATTTM Integration Vector Design & Construction |
Stage 3: Transfection of Targeting Vectors |
Stage 4: CAR-iPSC Expansion & Confirmation |
Stage 5: CAR-NK Differentiation |
Stage 6: Custom Downstream Assay Services |
|
|
|
|
|
|
Available Off-the-shelf Control Lines: TARGATT™ CD19-CAR-iNK Control Line & TARGATT™-iNK Control Line - Inquire
Products and Services
Technical Details

At Applied StemCell, we used our TARGATTTM Master iPSC Line to insert CAR and generate CAR-iNK cells. The TARGATTTM iPSCs hold an integrase recognition landing pad at a safe harbor locus. When they are used with an “attB” containing donor plasmid, integrase expression enables the site-specific integration of a large transgene with high efficiency. With this technology, ASC successfully inserted CAR into its TARGATTTM Master iPSC Line. An integration efficiency >40% was observed, and flow cytometry data confirmed CAR expression. Our team is currently working on differentiating the CAR-positive iPSCs into NK cells.
TARGATT™ iPSC-iNK Platform
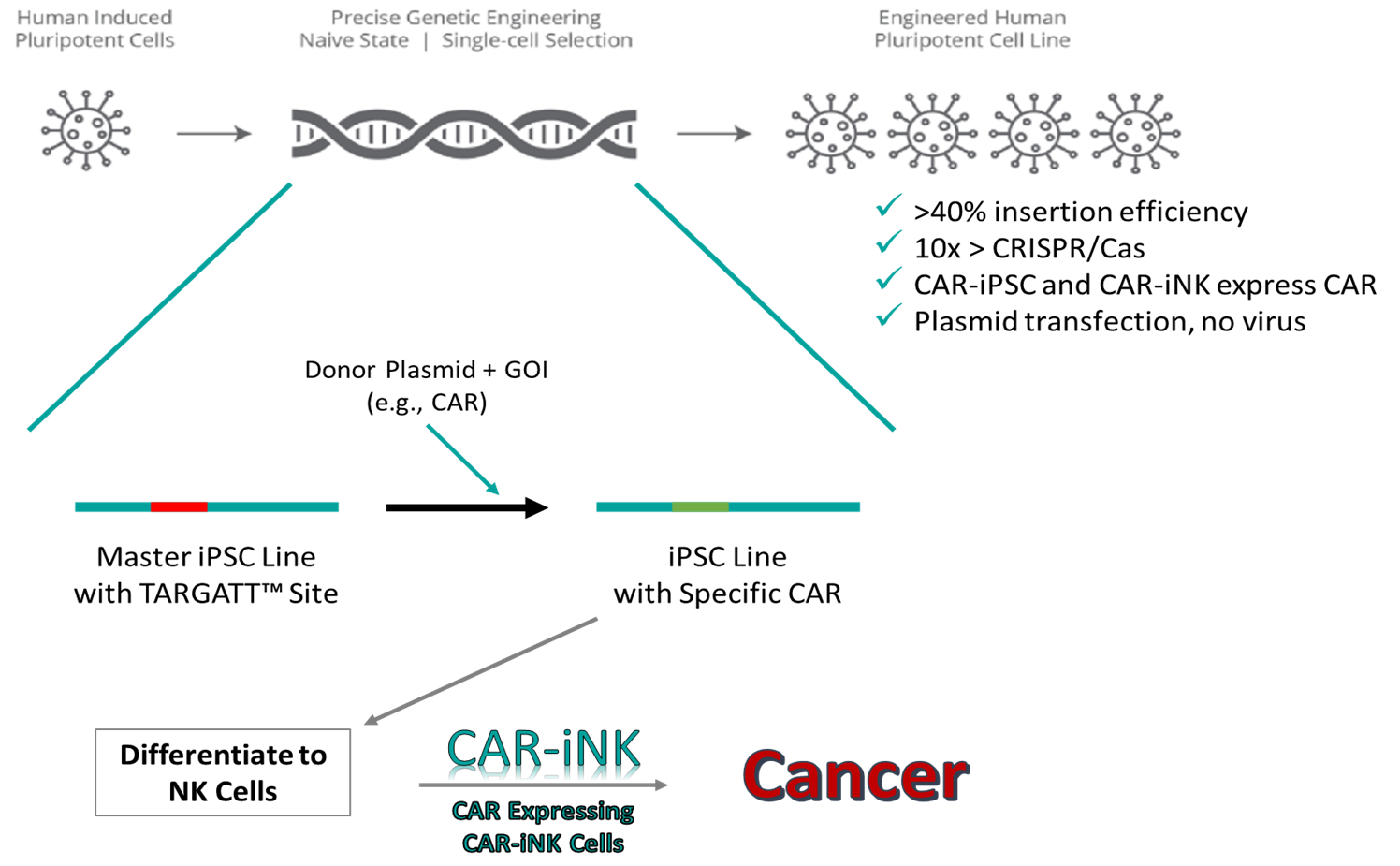
CAR Knock-In
ASC designed and constructed a TARGATTTM donor vector and performed transfection of the targeting reagents by lipofectamine. An integrase was used to catalyze the site-specific insertion of CAR (Cell-therapy gene) into the landing pad of the TARGATT™ Master iPSC Line.
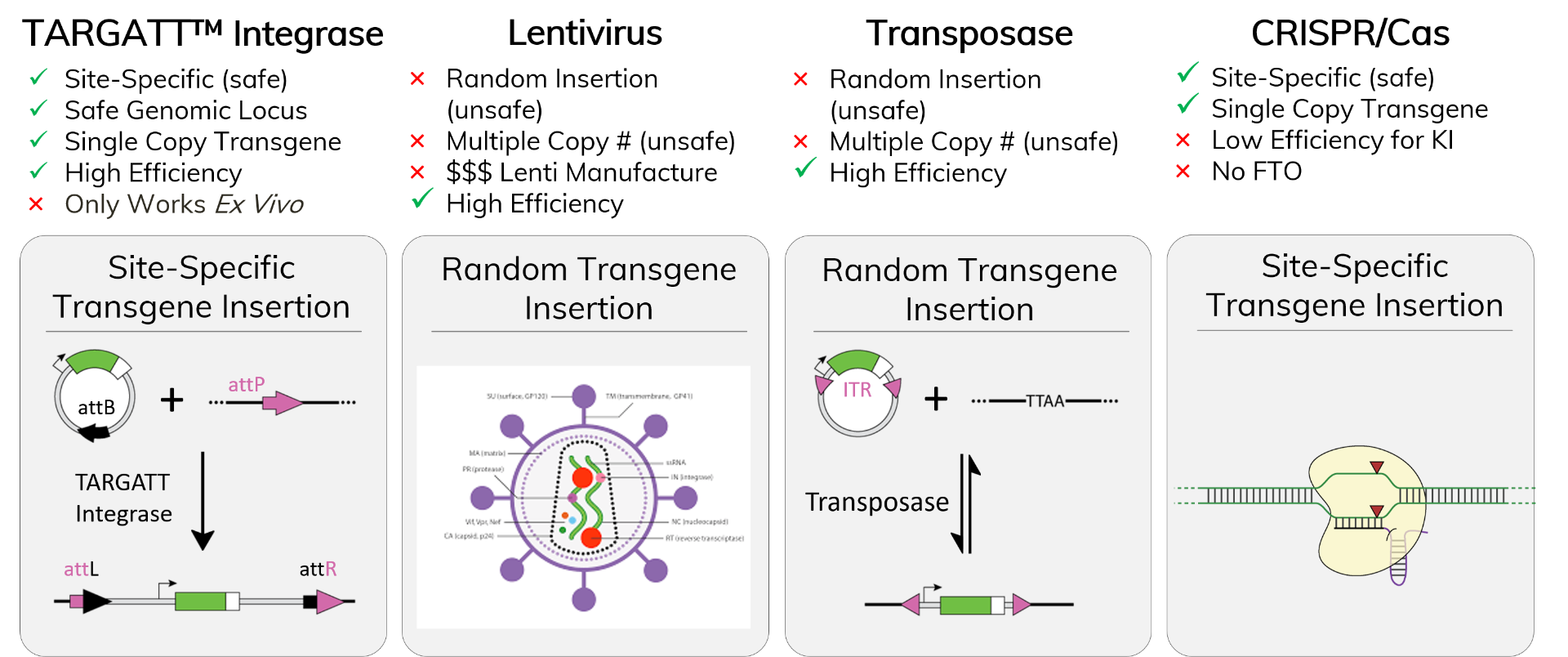
TARGATT™-Mediated Genome Engineering
Advantages vs. Other Systems
Confirming CAR Positive iPSCs
Our scientists screened several iPSCs and confirmed CAR expression by flow cytometry using antibodies labeled with a red fluorescent molecule.
CAR-iPSCs Express CAR
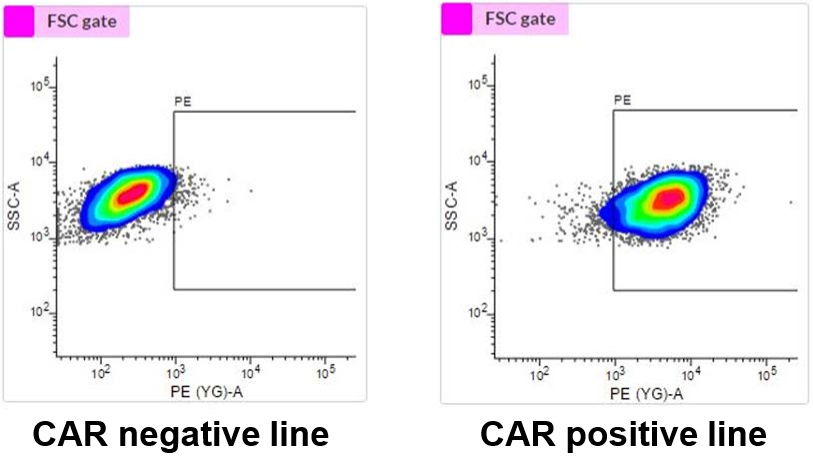
Figure 1: Flow cytometry analysis of iPSC lines screened for surface expression of CAR. Monoclonal antibodies labeled with a red fluorescent molecule were used for screening. During the screening process, an iPSC line that expressed CAR was identified. (Images: Left: CAR negative iPSCs; Right: CAR positive iPSCs)
CAR-iPSC Differentiation to Natural Killer Cells
Since CAR expression was confirmed, ASC is using its optimized differentiation protocols to produce high-quality NK cells from the CAR-iPSCs.
Characterization of the ASE-9708 NK Cells
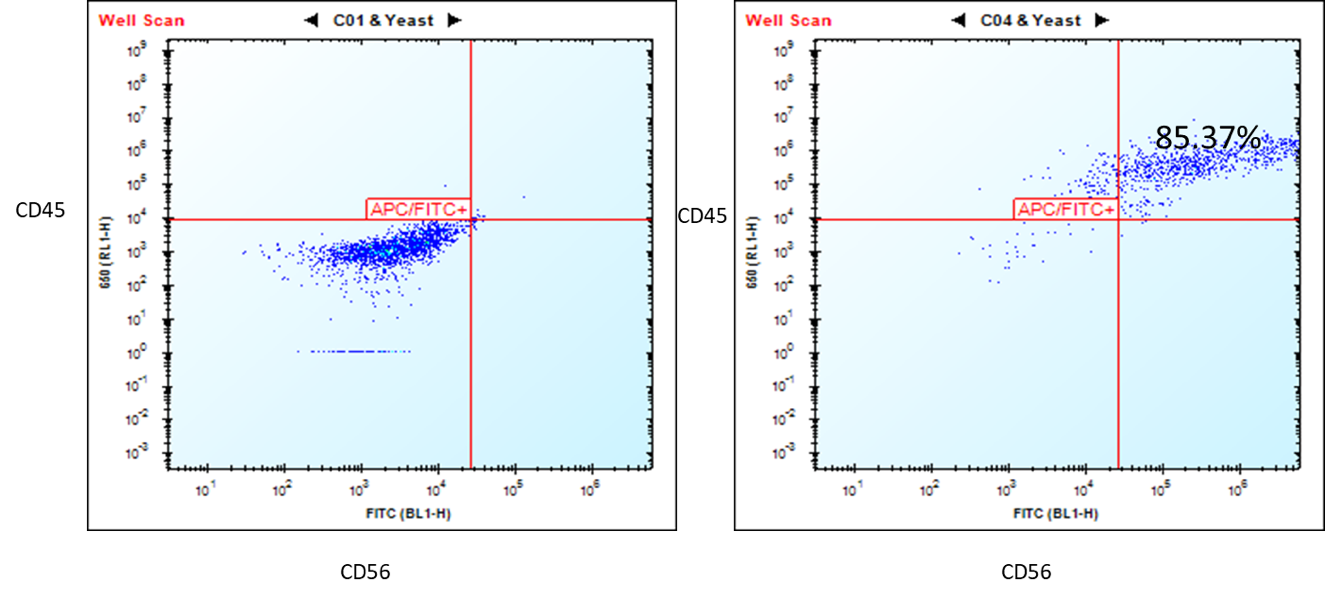
Figure 2. Flow cytometry analysis of ASE-9708 iPSC-derived NK cells for NK cell biomarkers. Cryopreserved NKs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in NK culture media. The cells were stained with NK cell markers, CD45 and CD56 at day 2. Left: Isotype control antibodies. Right: CD45/CD56.
Our team will work with you every step of the way. During your free consultation, we will gather the necessary information for your detailed project proposal that will be designed by our scientists. You will receive a personalized experimental/project outline and project schedule. Once approved, you provide your CAR genetic material or information, and we take care of the rest. In just a few months, you will receive the high-quality iPSC-derived CAR-iNK cells you need for your proof-of-concept cancer immunotherapy research. Contact ASC today to schedule your free consultation.
Support Materials
FAQs
Do you provide off-the-shelf positive and negative control lines?



