Newsletter
Astrocytes Differentiation Service
Astrocytes, the most abundant glial cells found in the central nervous system and spinal cord, play a critical role in neuronal development, neuronal metabolism, neurotransmitter synthesis, and synaptic function as well as repair and neurogenesis during CNS and spinal cord injury. Astrocyte dysfunction has been implicated in neurological disorders such as Parkinson’s disease, Alzheimer’s disease, Amyotrophic lateral sclerosis, and Huntington’s disease. iPSC-derived astrocytes provide a readily sourced, consistent, and biologically relevant alternative to primary astrocytes for the study of synaptic transmission and plasticity in normal CNS function and disease progression.
Human iPSC-derived astrocytes are powerful in vitro tools for developing physiologically relevant models of human CNS and diseases. These iPSC-derived astrocytes recapitulate the morphological and functional properties of primary cells with the advantages of cell culture. Applied StemCell provides custom service to differentiate your iPSCs into ready-to-use, high-purity astrocytes:
- Robust, mature astrocytes with the morphology of primary astrocytes
- Cells express key astrocyte-specific markers
- Differentiate your healthy, disease or engineered iPSCs
- Control “Master” iPSCs and iPSC generation services are available for deriving control astrocyte lines
- Fast Turnaround: 2 months
- GMP iPSC Differentiation Services Available >> Learn More
| Astrocytes | |
| Biomarkers | GFAP |
| S100beta | |
| Other markers available upon request |
Optional! Cell Line validation and drug screening services are available.
Products and Services
Case Studies
Astrocyte Differentiation Service
Case Study 1:
Project Description:
- Differentiation of Customer Provided iPSCs to Astrocytes
Timeline:
- iPSC Recovery & Expansion
- Pathogen Screening
- Astrocyte Differentiation
- Antibody Staining (GFAP+, S100beta) (Flow Cytometry is Available if Inquired)
Turnaround time: 3-4 months
Deliverables:
- 5 million cells; 1 million/vial
- Final Report with QC Data
- QC Data Includes:
- Human Pathogen Screening Result for the Parental Line
- Recovery Test
- Mycoplasma Test
- Antibody Staining (GFAP, S100beta)
- QC Data Includes:
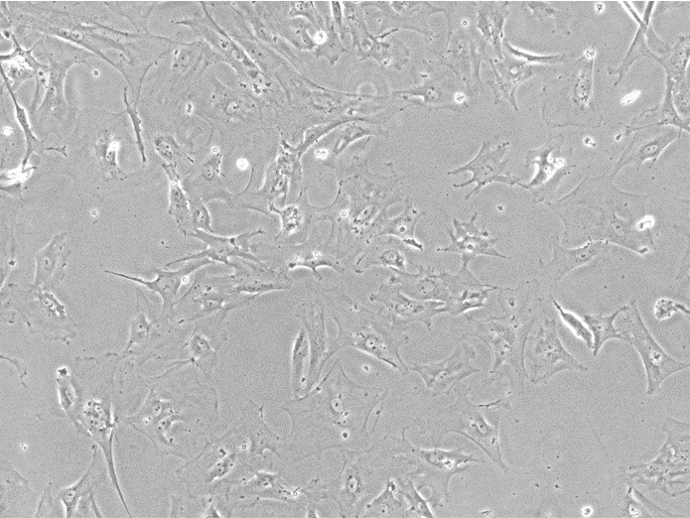
Figure 1. Bright Field Image of iPSC-derived Astrocytes From Customer-provided iPSCs.
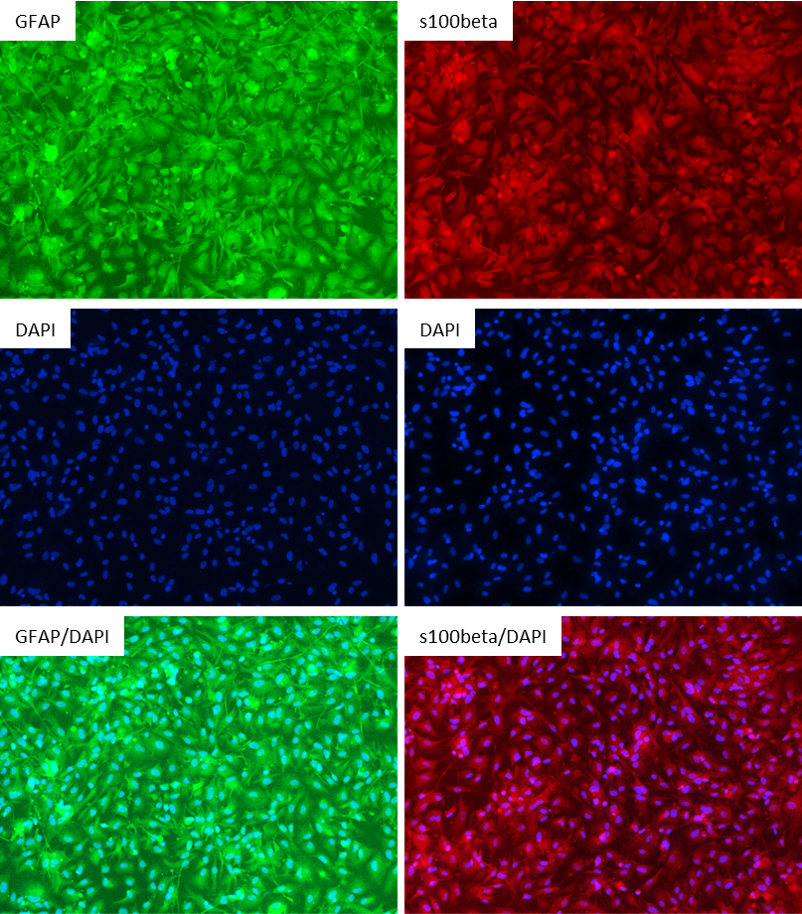
Figure 2: Antibody Staining of the iPSC-derived Astrocytes. The astrocytes differentiated from the customer's iPSC line were stained with the astrocyte markers GFAP and s100beta. Left Three Images: Top Image: GFAP (Green), Middle Image: DAPI (Blue), Bottom Image: GFAP (Green) & DAPI (Blue); Right Three Images: Top Image: s100beta (Red), Middle Image: DAPI (Blue), Bottom Image: s100beta (Red) & DAPI (Blue)
Case Study 2:
Project Description:
- To differentiate astrocytes from Applied StemCell’s NIST Control Line, ASE-9211
Timeline:
- iPSC Recovery & Expansion
- Astrocyte Differentiation
- Antibody Staining (GFAP+, S100beta) (Flow Cytometry is Available if Inquired)
Turnaround time: 3-4 months
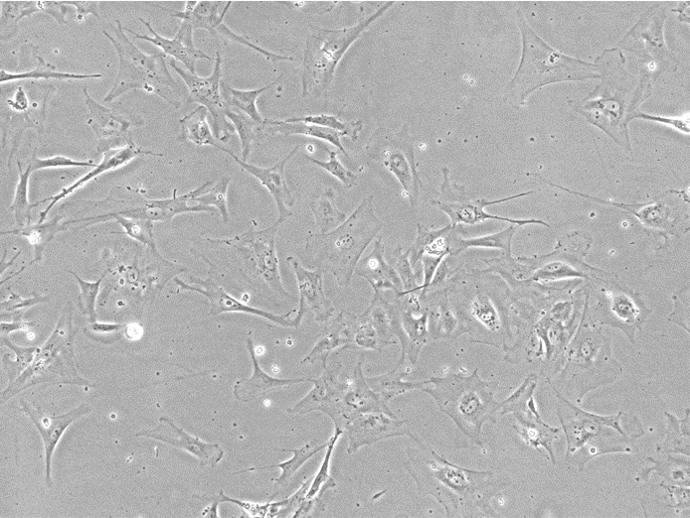
Figure 1. Bright Field Image of iPSC-derived Astrocytes from Applied StemCell’s (ASC's)Control Line, ASE-9211.
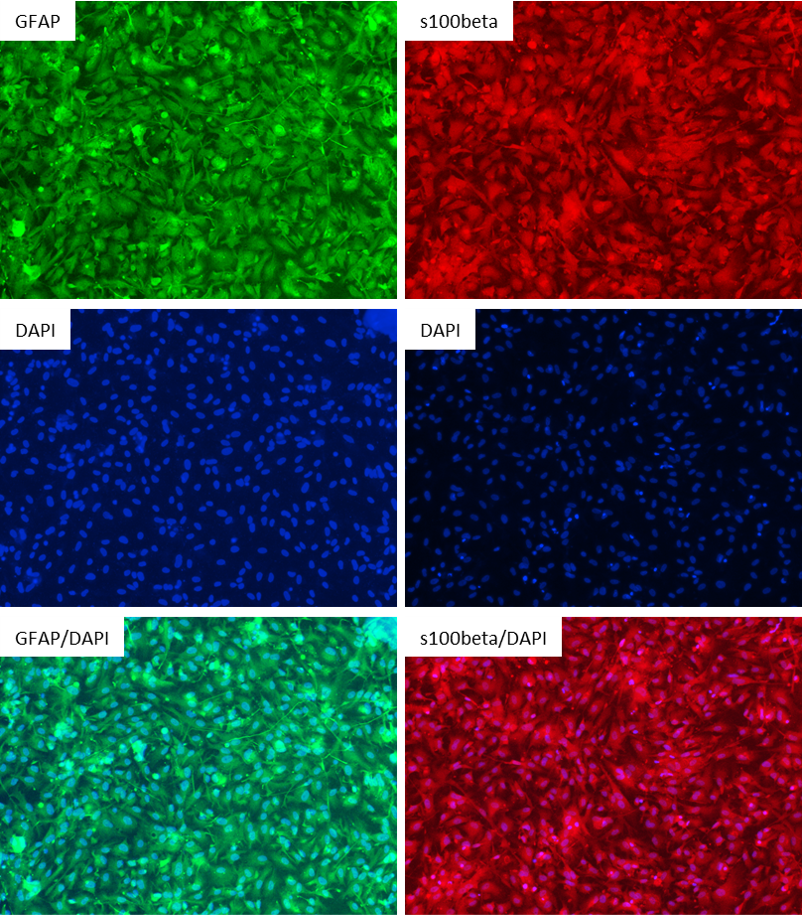
Figure 2: Antibody Staining of the Astrocytes differentiated from the NIST Control Line, ASE-9211. The astrocytes differentiated from the ASC's iPSC line were stained with the astrocyte markers GFAP and s100beta. Left Three Images: Top Image: GFAP (Green), Middle Image: DAPI (Blue), Bottom Image: GFAP (Green) & DAPI (Blue); Right Three Images: Top Image: s100beta (Red), Middle Image: DAPI (Blue), Bottom Image: s100beta (Red) & DAPI (Blue)
Case Study 3
Project Description:
- Astrocyte Differentiation from Control Human iPSC
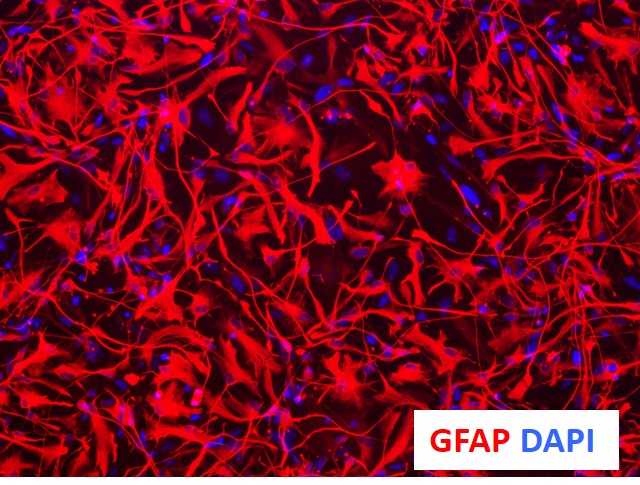
Figure 1. Immunocytochemical characterization of astrocytes derived from NSCs using proprietaryprotocols and optimized media yield >90% GFAP+ cells (astrocyte marker) and <1% Tuj1+ cells (neuronal marker).
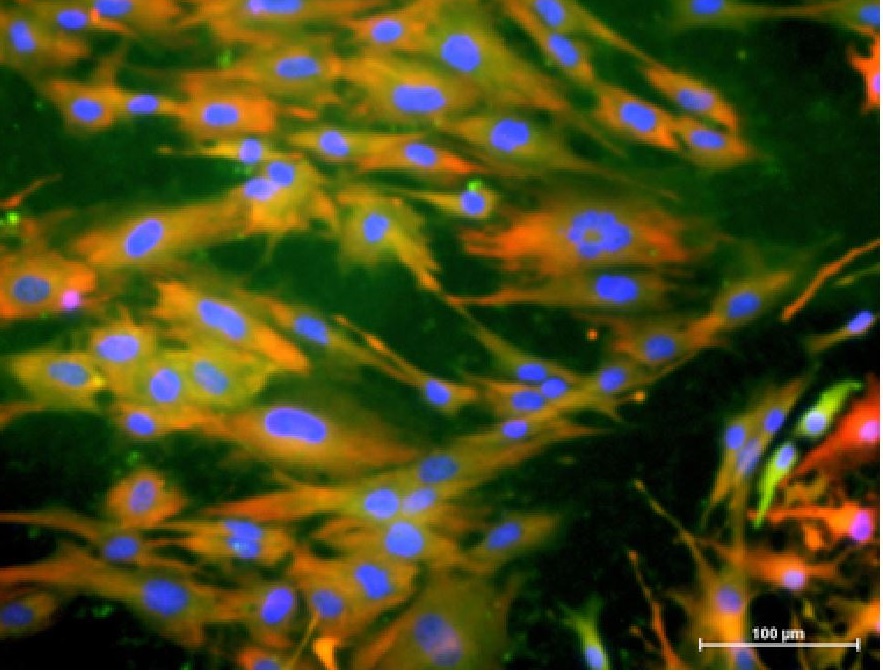
Figure 2. Cytoplasmic co-localization of astrocyte markers, GFAP (green) and S100b (red), and nucleus-staining marker, Hoechst (blue).
Application Notes
Benefits and advantages of iPSC differentiation to neurons and glial cells:
- iPSC-derived neurons and glial cells are genetic and physiologically relevant in vitro models to study neural development and associated disorders: congenital disorders, neurodegenerative disorders and brain tumors.
- Avoid genetic variability; generate isogenic neurons and glial cells in cell culture formats that are reproducible and scalable
- Generates a valuable model for identifying new targets for neuro-regeneration as opposed to treatments limiting to symptomatic relief or delaying disease progression.
- Allows for future adaptation of technology for regenerative medicine and cell therapy in humans for the treatment of Parkinson’s disease, Lou-Gehrig disease (ALS), Huntington’s disease, and spinal cord injury among other diseases.
- Differentiation of genome engineered in iPSCs (mutation introduction or correction) to neurons, offers an isogenic source of control-disease cell lines for basic research, drug development, hard-to-model neurological disorders, and potentially for gene therapy.
Applications:
- Co-culture with neurons for advanced, in vivo-like cell line models
- Neurotoxicity screening
- Disease modeling
- High throughput drug discovery and drug screening applications
- Drug neurotoxicity screening applications
FAQs
Will the iPSC-derived astrocytes be provided as mature or precursor cells?
Is there a separate medium for maintaining iPSC-derived astrocyte cells in culture?
Do you characterize astrocyte precursor cells (APCs) using CD44 or A2B5 staining?
Will you be able to provide astrocyte precursor cells (APCs) be passaged?



