Newsletter
NK Cell, T Cell & CD34+ HPC Differentiation
iPSC Differentiation to Immune Cells/ Blood Cells
iPSC-derived T cells, natural killer cells (NK) cells, hematopoietic progenitor cells (HPCs) CD34+, dendritic cells, and others provide a next-generation toolset for understanding cancer and disease pathology as well as developing successful immunotherapies (adoptive therapies like CAR-T, CAR-NK, TCR-T, and more). Applied StemCell, with its world-class comprehensive stem cell platform which includes iPSC generation, genome editing, and differentiation, can differentiate your iPSCs into CD34+ hematopoietic lineage progenitor cells and further into high-quality lineage-committed immune cells: CD8+ and CD4+ T cells, CD34+ HPCs, NK cell, dendritic cells, monocytes, and more. These cells are ideal for immunotherapy research for cancer and immune disorders as well as for the development of complex co-culture models to screen drugs and understand immune cell activation and function.
- Optimized & efficient differentiation protocols
- Our team can work with your iPSCs, one of ASC's control lines, or we can generate your iPSCs
- Well-characterized cells delivered in a few weeks
- Affordable Prices
- Standard or Customizable Service Options
- GMP iPSC Differentiation Services Available >> Learn More
| HPCs | NK Cells | T Cells | |
| Biomarkers | CD34 | CD56 | CD3 |
| CD43 | CD45 | CD4 | |
| CD8 |
Fast Turnaround:
- HPCs: 3 weeks
- NK Cells: 2 months
- T Cells: 2 months
Do you need iPSCs to genetically modify and further differentiate into immune cells? We offer fully customizable iPSC generation from human or non-human tissue samples, and our genome editing experts can use CRISPR or TARGATT™ to genetically alter the iPSCs to fit your project requirements. If you need to expedite your experimental timeline, ASC also offers off-the-shelf HPCs, NK cells, and T cells. Contact us today to find the best solution for your immunotherapy projects.
________________________________________________________________________________________________________________________________________
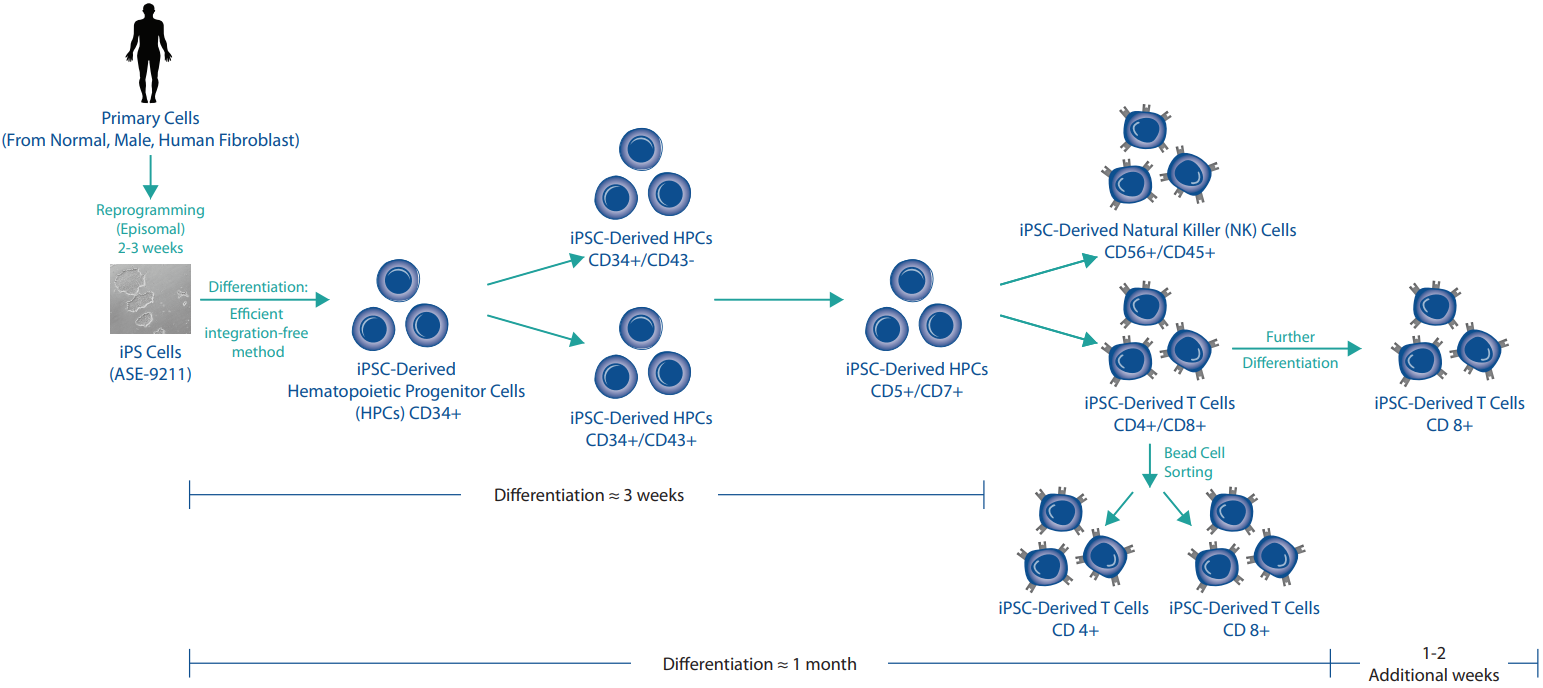
iPSC Differentiation
Figure 1: iPSC Differentiation Process.
Products and Services
Case Studies
Case Study 1
Characterization of the ASE-9708 NK Cells
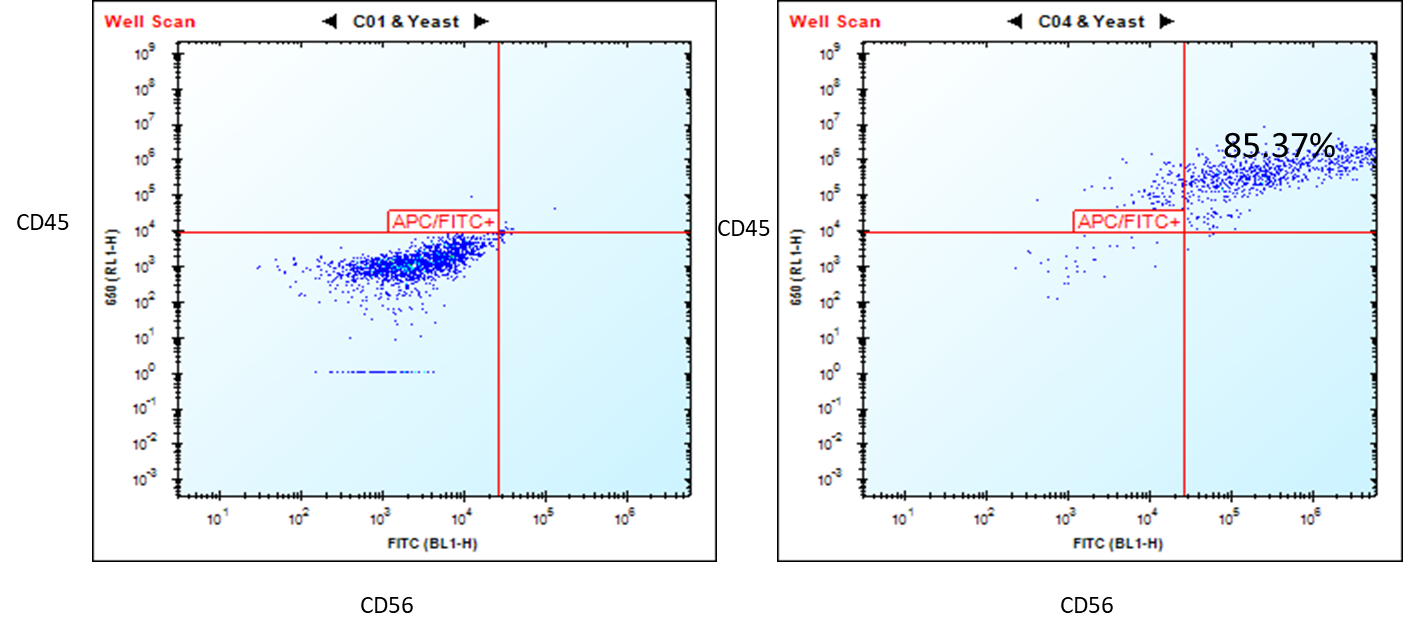
Figure 1. Flow cytometry analysis of ASE-9708 iPSC-derived NK cells for NK cell biomarkers. Cryopreserved NKs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in NK culture media. The cells were stained with NK cell markers, CD45 and CD56 at day 2. Left: Isotype control antibodies. Right: CD45/CD56.
Case Study 2
Characterization of the ASE-9720 T Cells
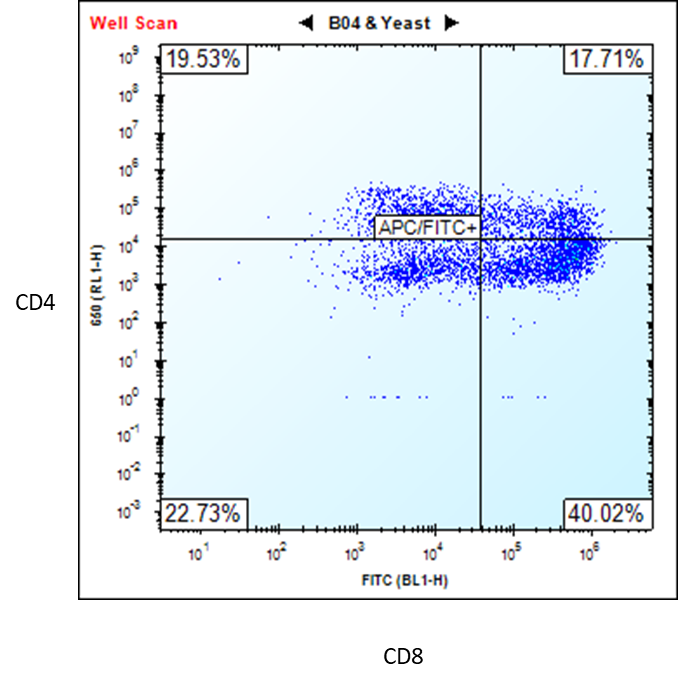
Figure 2. Flow cytometry analysis of ASE-9720 iPSC-derived T cells for T cell biomarkers. The T cells,
differentiated from Applied StemCell’s control iPSC line, ASE-9211, were stained with T cell markers CD4 and
CD8. (Premature T Cells CD4+/CD8+ = 17.71% and Mature T Cells CD8+ = 40.02%) *
*Upon request, the T cells can be purified by FACS or MACS (single positive or double positive). We can also further differentiate the cells to obtain a higher percentage (≥80) of
mature CD8+ T cells.
Case Study 3
Characterization of the ASE-9718 Hematopoietic Progenitor Cells
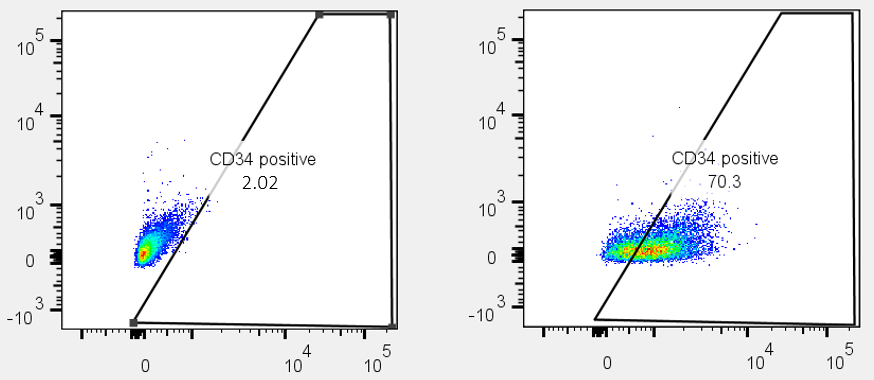
Figure 3. Flow cytometry analysis of ASE-9718 iPSC-derived Hematopoietic Progenitor Cells for an HPC biomarker. Cryopreserved HPCs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in HPC culture basal media. The cells were stained with HPC cell marker, CD34 (right panel) or isotype control (Left panel).
Case Study 4
Characterization of the ASE-9719 Hematopoietic Progenitor Cells
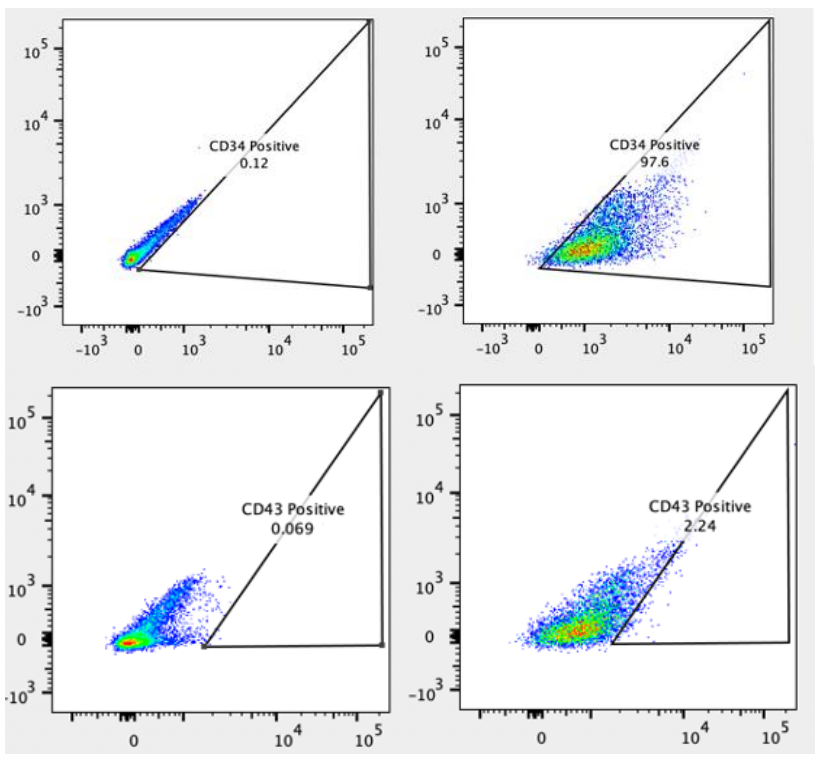
Figure 4. Flow cytometry analysis of ASE-9719 iPSC-derived Hematopoietic Progenitor Cells for HPC biomarkers. Cryopreserved HPCs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in HPC culture basal media. The cells were stained with HPC markers, Top: CD34 (Right panel) or isotype control (Left panel), Bottom: CD43 (Right panel) or isotype control (Left panel).
Application Notes
The development of cellular immunotherapy (such as CAR-T, CAR-NK cell therapies, and other adoptive cell transfers) is gaining momentum for targeted treatment of cancer and immune disorders, and in understanding the role of immune response in the pathology of diseases. Therefore, in-depth knowledge of the hematopoiesis of immune cells, their activation, and function is critical to understanding disease pathology and developing safe and effective therapies. Current processes for developing such cell-based therapies are complex and involve: harvesting of patient (autologous) cells, handling and processing patient-derived primary cells, including genome engineering the cells to target specific antigens, and re. And, result in only a single dose of the product being manufactured that is heterogeneous in its characteristics and very expensive to develop and engineer.
The revolutionary iPSC technology may provide a reliable and standardizable starting material to generate various immune cells for therapeutic purposes, to screen drugs, and for disease modeling; and thus, overcome the difficulties of sourcing patient’s primary cells, genome engineering these notoriously difficult to engineer cells, and avoid the variance in cellular characteristics associated with current methods:
- A more physiologically relevant cell line model combining the advantages of cell culture and primary cells
- Unlimited self-renewal potential of iPSCs would assure a steady and consistent supply of cells
- Single cell clonability for a homogeneous population of cells
- CRISPR and genetic engineering amenability to engineer antigen-specific cells for targeted therapy
- Potential to differentiate into many different cell types with same genetic background, including iPSC-derived CD34+ progenitor cells, CD4+T helper cells, CD8+ cytotoxic T cells, natural killer (NK) cells, dendritic cells, macrophages and more
- Full characterization possible
- Scalability for mass production
- Potential for developing into cryopreserved, off-the-shelf therapeutic products
The iPSC-based cellular immunotherapy has great potential to make the successful bench-to-bedside translation a reality.
Applications for iPSC-derived immune cells include:
- Disease modeling for cancer and autoimmune disorders
- iPSC-derived antigen-specific CAR-T/CAR-NK research
- Adoptive transfer and other cell-based therapies
- Antibody discovery
- Drug target discovery and drug screening
- Cell line models for drug toxicity screening
- Cell-based therapies
- Co-culture cells of different lineages to model in vivo heterogeneity of human immune system and cancer pathology
- Multiplexing target attributes
T lymphocytes (T cells): With the FDA approval of two novel T cell-based therapies for treating cancer, Kymriah™ and Yescarta™, the race for immunotherapies for different types of cancer and immune system-based disorders has intensified. These are a type of adoptive cell transfer (ACT) therapies called chimeric antigen receptor-T cell (CAR-T) where a patient’s (autologous) T cells are genetically engineered ex vivo, to recognize and kill tumor-specific antigens expressed on the surface of tumor cells. They leverage the central role played by T lymphocytes in the immune response to treat cancer. Another type of cytotoxic T lymphocyte cell therapy includes directly delivering ex vivo engineered autologous T cell receptors (TCR-T) that recognize tumor-specific intracellular proteins presented as protein fragments by the major histocompatibility complex (MHC) class I proteins.
Although a great success initially in cancer remission and treatment, there are limitations in the technology such as: limited to autologous (patient) T cells, loss of naïve T cells and persistence of long-term immunologic memory as T cells acquire effector functions, and T cell exhaustion due to repeated activation of T cells in cancer patients which severely reduces the amount of cells that can be harvested from the patient. Such limitations may be overcome by using T cells differentiated ex vivo from iPSCs reprogrammed from patient cells. These T cells have rejuvenated T cell characteristics and can be produced in high numbers with consistent and reliable quality. Additionally, iPSCs can be genetically engineering more efficiently than primary T cells, and can be used for generating CAR-T and TCR-T cells for immunotherapy applications.
Natural Killer (NK) cells are a type of lymphoid cells that originate from the same progenitor as T cells and B cells. They are an important part of the innate immune response. NK cells are activated in response to interferons or macrophage-derived cytokines. They recognize “non-self” cells without the need for antigen presentation or recognition, executing a rapid immune reaction. The broad cytotoxicity and rapid apoptosis induced by the NK cells helps contain virus-infected cells and controlling early signs of cancer while the adaptive immune response is activated to produce cytotoxic T cells to clear the antigens. With the improving success rate of CAR-T cell therapy, scientists are exploring the NK cells to engineer them for CAR-NK cell therapy to target cancer cells. CAR-NK cells have certain advantages over CAR-T cells in that they retain their natural tumor recognition and killing ability; they do not require strict HLA matching and lack the potential to cause graft-versus-host disease (GvHD), they do not have the same safety concerns as seen with CAR-T cells such as cytokine release syndrome, and therefore they can be generated from allogenic donors and possibly developed as off-shelf therapeutic products.
However, purification of NK cells from allogenic hosts is critical since residual T and B cells may cause GvHD and other complications. Primary NK cells are difficult to harvest, purify, and transfect/transduce, and result in a heterogenous population. They are also difficult to standardize due to the heterogeneity in starting material from different donors. As well, the generation of large quantities of highly pure NK cells requires an extended manufacturing process which can compromise recovery of NK cells, their viability, and potency. Therefore, iPSC-derived NK cells offer a consistent and renewable starting material to provide standardizable NK cells without the dependency on donors and complex harvesting and purification processes. They offer a method to generate homogenous populations of CD56+ CD45+ NK cells and subsequently to generate CAR-NK cells for targeted allogenic cancer immunotherapy, even solid tumors.
Morris, E. C., & Stauss, H. J. (2016). Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood, 127(26), 3305-3311.
Kawamoto, H., Masuda, K., Nagano, S., & Maeda, T. (2018). Cloning and expansion of antigen-specific T cells using iPS cell technology: development of “off-the-shelf” T cells for the use in allogeneic transfusion settings. International journal of hematology, 107(3), 271-277.
Minagawa, A., Yoshikawa, T., Yasukawa, M., Hotta, A., Kunitomo, M., Iriguchi, S., ... & Kawai, Y. (2018). Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell stem cell, 23(6), 850-858.
Natural killer cells for cancer immunotherapy: a new CAR is catching up. (). EBioMedicine, 39, 1–2. doi:10.1016/j.ebiom.2019.01.018.
Zeng, J., Tang, S. Y., Toh, L. L., & Wang, S. (). Generation of "Off-the-Shelf" Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells. Stem cell reports, 9(6), 1796–1812. doi:10.1016/j.stemcr.2017.10.020.