Newsletter

Our iPSC reprogramming service offers a method of generating patient-specific stem cells of any lineage without using embryonic materials. We utilize various strategies to improve reprogramming technologies, including chemical and transgene reprogramming. Our protocols have been streamlined for efficient iPSC generation using viral vectors, DNA (plasmid) and mRNAs. We offer comprehensive reports suitable for publications with each of our services.
We offer reprogramming using various vectors, including retrovirus, lentivirus, episomal plasmid, and direct delivery of synthetic mRNAs. W When deciding on a reprogramming method, we consider the cell being reprogrammed and the ability of the reprogramming method to adequately reprogram this cell type. We also assess whether the presence of integrated sequences in the iPSCs will hinder downstream application.
Let the iPSC experts generate high-quality iPSCs and derived physiologically relevant cell line models from your healthy/disease samples. With our optimized reprogramming protocols and comprehensive characterization services, we deliver iPSCs ideal for your basic research, drug discovery, drug screening, and preclinical cell regeneration projects:
Not only can ASC further characterize your iPSCs, but our experts can also genetically engineer your iPSCs using CRISPR/Cas9 or TARGATT™ and differentiate the iPSCs to the cell type of your choice, including NK cells, T cells, astrocytes, cardiomyocytes, and more.



GMP iPSC Generation and Additional Services Available >> Learn More
Applied StemCell (ASC) has provided stem cell and genome editing services for over 13 years, and we have worked with researchers all across the globe to engineer over 500 unique cell line models. As one of the earliest providers of CRISPR/Cas9 genome editing services, ASC has the experience and optimized protocols for Rapid Automated Cell Line Editing (RACE™) in induced pluripotent stem cells (iPSCs)!
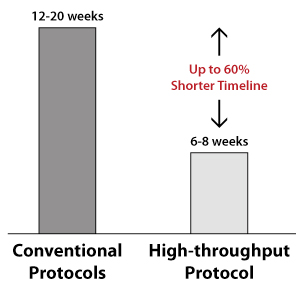
With our well-established high-throughput protocols, ASC's experts can produce any complex or mainstream genetic modification in your healthy or diseased iPSCs for your basic research, disease modeling, tissue engineering, regenerative medicine, or cell-based therapy research. Leverage our efficient CRISPR genome editing method to obtain your CRISPR-engineered iPS cells in just a few weeks.
High success rate: >98% projects completed to customer’s specifications
ASC can genetically modify your healthy or diseased iPSCs; control lines are available
Single cell cloning (clonal isolation)
Homozygous or Heterozygous
Automated processes for consistency and high throughput scalability
Pluripotency maintained throughout genome editing process using high-end cell culture reagents and protocols
ASC is a one-stop-shop for all your iPSC service needs. We are one of the few providers of integrated upstream iPSC generation & downstream differentiation and assay development services. If you are looking to engineer iPSCs in a GMP setting, we invite you to read more about our new GMP-grade iPSC service offerings.
Save Your Time, Money & Effort! Leverage our extensive expertise in CRISPR/Cas9 genome editing technologies to generate genetically modified mouse, rat, cancer and stem cell lines, with a variety of modifications in a targeted gene of interest. As one of the earliest licensees of CRISPR/Cas9 technology, we have genetically engineered > 1800 unique cell line and animal models for disease modeling, functional genomics, target identification, antibody validation, and validation for drug discovery and screening, and more. We offer affordable, comprehensive custom service with a fast turnaround time to meet the exact requirement of your projects. You can also combine it with our downstream custom assay services for a seamless project workflow.


iPSC differentiated cell lines offer the convenience of cell line models with the biorelevance of primary cells but without the sourcing difficulties and lot-to-lot variability issues associated with primary human cells. Applied StemCell (ASC) offers comprehensive service for iPSC differentiation to lineage-committed cell types thereby expanding the scope of your research, drug discovery, or drug screening projects.
Our experts can differentiate your iPSCs or you can select from our inventory of well-characterized iPSC control lines. ASC even offers upstream iPSC generation from human or non-human samples and high-throughput CRISPR genome editing services. To learn more about our affordable, custom iPSC services contact us today.
GMP iPSC Differentiation & Additional Services Available >> Learn More
Applied StemCell’s (ASC) proprietary TARGATT™ technology, enables fast and site-specific, stable integration of large DNA fragments (up to 20 kb) into an intergenic, transcriptionally active safe harbor locus with very high efficiency. The preselected locus is engineered to contain an "attP" integrase recognition landing pad where single-copy gene integration occurs when used in conjunction with an “attB” containing donor plasmid and integrase expression.
The TARGATT™ gene editing platform is versatile and can be used for the development of large fragment knock-in cell lines, bioproduction, and library construction. This technology circumvents problems associated with random integration such as position effect, and gene silencing or instability due to the integration of multiple copies of the transgene.
ASC can accurately and efficiently engineer the necessary landing pad into the cell line of your choice. Ready-to-use TARGATT TM Master Cell Lines (iPSC, HEK293, and CHO) are also available for integration of your gene of interest (GOI) at a preselected locus that has been tested for uniformed, high gene expression. Contact us today to schedule your free consultation!



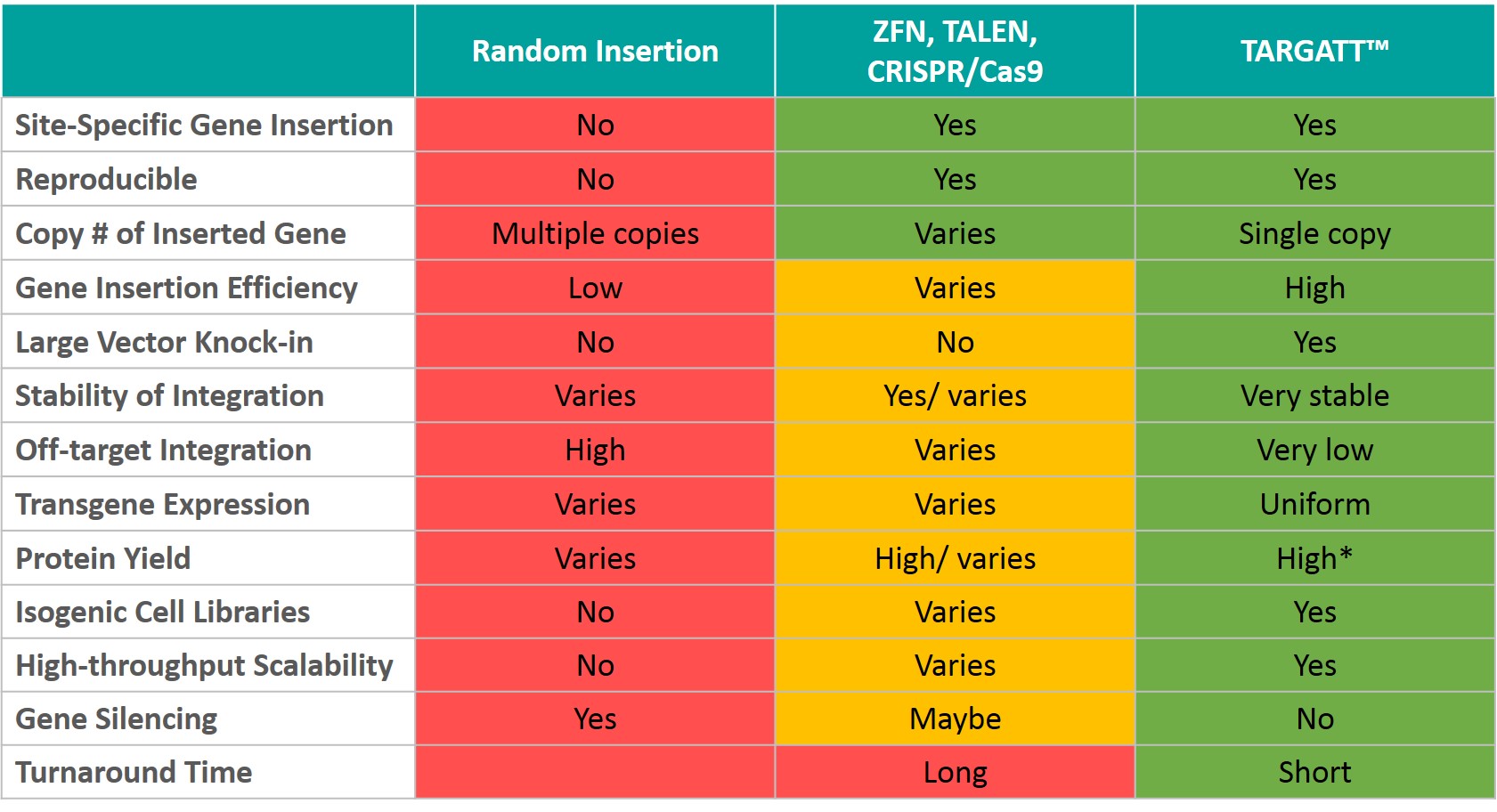
Technology Comparision
Stringent molecular and functional assays are necessary to evaluate pluripotency and to rule-out genetic aberrations due to reprogramming and stress from in vitro culture in iPSC lines. Loss of genetic integrity can affect desired cellular phenotype and compromise interpretation and translation of results to a clinical setting.
ASC offers comprehensive services needed to completely characterize your human and mouse pluripotent stem cell lines (PSCs):
Our comprehensive start-to-finish ipsc and stem cell platform offers downstream services to advance your iPSC-based projects to the next stage. We offer preclinical CRO solutions for drug screening, protocol development for cell regeneration and adoptive transfer model, CAR-T research, universal and immunocompatible cell line, and more. We can customize every stage of your project and find the best fit to suit your research needs.
ASC received its Drug Manufacturing License from the California Food and Drug Branch in 2021. Since then, iPSC processes have been established for cell banking and iPSC reprogramming, genome editing (CRISPR and TARGATT™), and differentiation that are compliant with FDA (US) and EMA (EU) regulations. As a CRO/CDMO committed to providing high-quality services and products, we follow quality control protocols based on ICH-Q5A/D guidelines for the development of safe iPSC and iPSC-derived products in our ISO Class 5 and 7 (Class 10,000 and 100) clean room.
In addition to our cGMP-compliant iPSC generation service, we offer well-characterized, ready-to-use GMP-grade iPSCs (Cord Blood CD34+, Male) and cGMP-Compliant TARGATTTM Master iPSCs that can be engineered to express almost any gene of interest (e.g., CAR genes). The genome-edited cells can be further differentiated into various cell types, including NK cells, T cells neurons, RPE cells, Cardiomyocytes, Neurons, and more. Our team of experts can even work with customer-provided cell lines. We currently offer:
Current Good Manufacturing Practice (cGMP):
Regulations ASC complies with as a cell processing GMP facility <Located in CA, USA>
Product QC:
As natural killer (NK) cell-based cancer immunotherapy research continues to grow, so has the number of potential chimeric antigen receptor (CAR)-NK therapies. Researchers continue to explore the cytotoxic capabilities of engineered NK cells, but several CAR-NK cell generation problems remain. For example, the CAR-NK cell development process takes several weeks, the patient’s NK cells may be weak and in a damaged state, or cancer cells may be created with random integration. Recent advancements in induced pluripotent stem cell (iPSC) technology have opened the door for the development of CAR-iPSCs that can be further differentiated into NK cells. These cells could potentially fix the current CAR-NK problems the research community is facing.
Applied StemCell (ASC) combined its optimized TARGATTTM gene editing and NK differentiation technologies to establish the TARGATTTM iPSC-iNK Platform. Our expert scientists can insert your specific CAR genetic material into our iPSCs at a safe harbor locus with an efficiency ~10x better than CRISPR and further differentiate your CAR-iPSCs to high-quality NK cells. Leverage our unlimited source of iPSCs and proprietary TARGATTTM technology to safely and efficiently produce the allogenic iNK cells you need to drive your research forward.
ASC Advantages
As a CRO/CDMO service provider that continuously works to improve and expand its technology, services, and products, we hope to have our new cGMP TARGATT™ iPSCs available for purchase early next year. For now, we offer you fully characterized cGMP grade iPSCs from CD34+ cord blood. Contact us today to learn more.
We understand working with iPSCs remains difficult, and you may encounter several challenges. At ASC our stem cell and gene editing experts are ready to help you every step of the way.
1. Send in your CAR design for review. Our experts can help you enhance your design.
2. Our team can design research and GMP projects in parallel OR generate a matching research grade cell line for preliminary testing.
3. Downstream Assay Services Are Available: cytotoxicity assays, in vivo testing, drug banking, and more - Inquire