Newsletter
iPSC-Derived T Cells, Natural Killer Cells & Hematopoietic Progenitor Cells
Using efficient integration-free, small molecule-based differentiation methods, ASC now supplies high-quality iPSC-derived T cells, natural killer (NK) cells, and hematopoietic progenitor cells (HPC) from human iPSCs (ASE-9211). The differentiated T cells, NKs, and HPCs recapitulate the phenotype and functional parameters of primary and in vivo cells. These iPSC-differentiated cells can be used as control lines to compare phenotype and functionality of patient-derived, genome-edited iPSC-derived cells for drug screening applications.
- Optimized & efficent differentiation protocols
- Well-characterized
- High-purity
- Affordable Prices
- GMP iPSC Products & Services >> Learn More
| HPCs | NK Cells | T Cells | |
| Biomarkers | CD34 | CD56 | CD3 |
| CD43 | CD45 | CD4 | |
| CD8 |
If you have your own iPSCs you would like to differentiate into HPCs, NK cells, or T cells, ASC provides standard and customizable iPSC differentiation services using your iPSCs or one of our iPSC control lines. We can even generate iPSCs from your tissue sample. Once you have the iPSC differentiated cells you required, our experts can help you develop any assay you may need for your research, including NK Cell Cytotoxicity Assays.
________________________________________________________________________________________________________________________________________
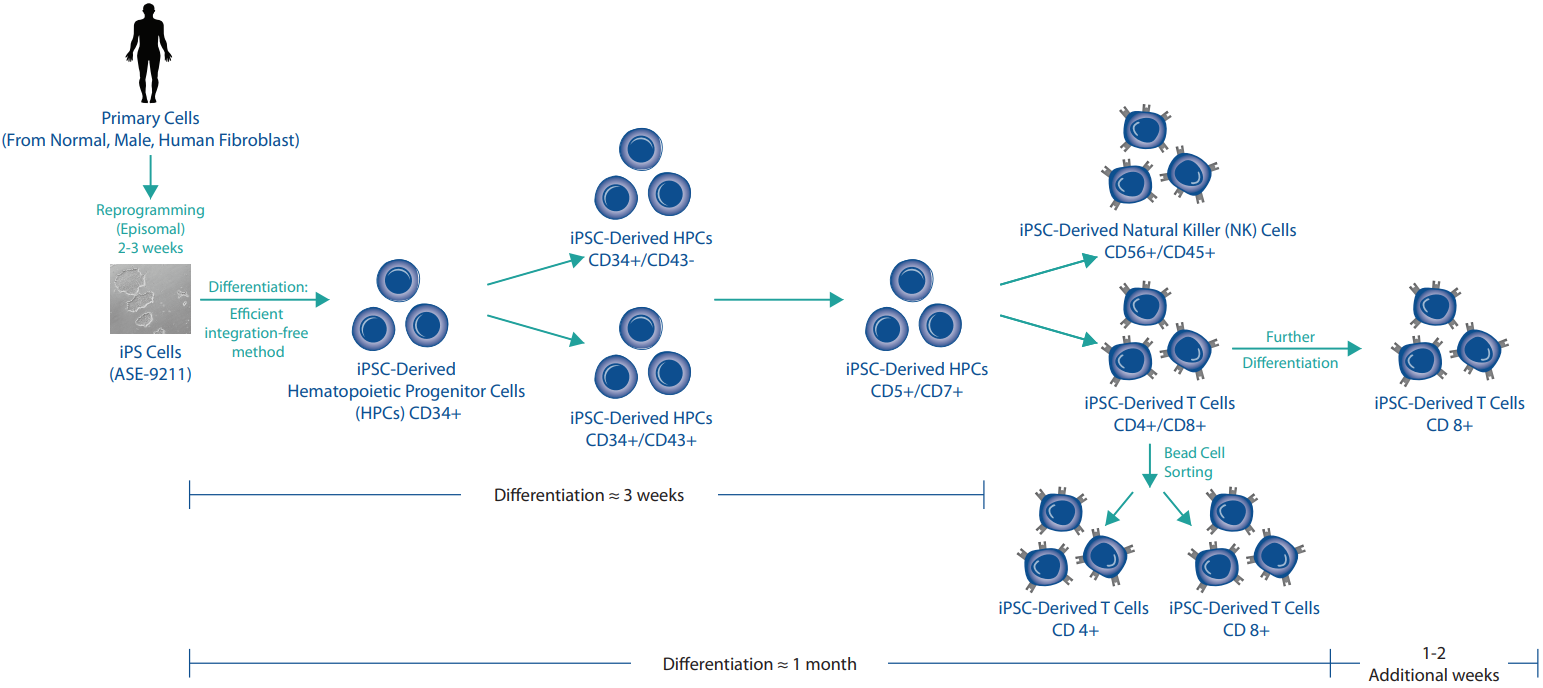
iPSC Differentiation
Figure 1: iPSC Differentiation Process.
Products and Services
Technical Details
Case Study 1
Characterization of the ASE-9708 NK Cells
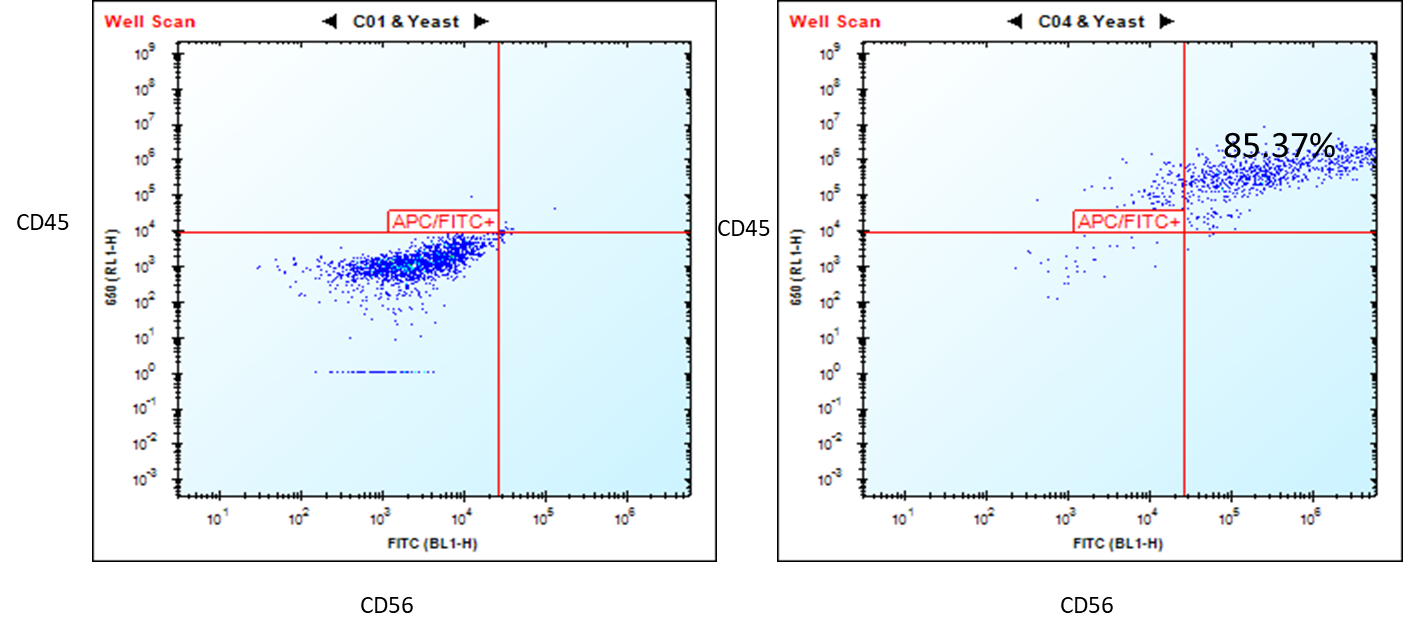
Figure 1. Flow cytometry analysis of ASE-9708 iPSC-derived NK cells for NK cell biomarkers. Cryopreserved NKs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in NK culture media. The cells were stained with NK cell markers, CD45 and CD56 at day 2. Left: Isotype control antibodies. Right: CD45/CD56.
Case Study 2
Characterization of the ASE-9720 T Cells
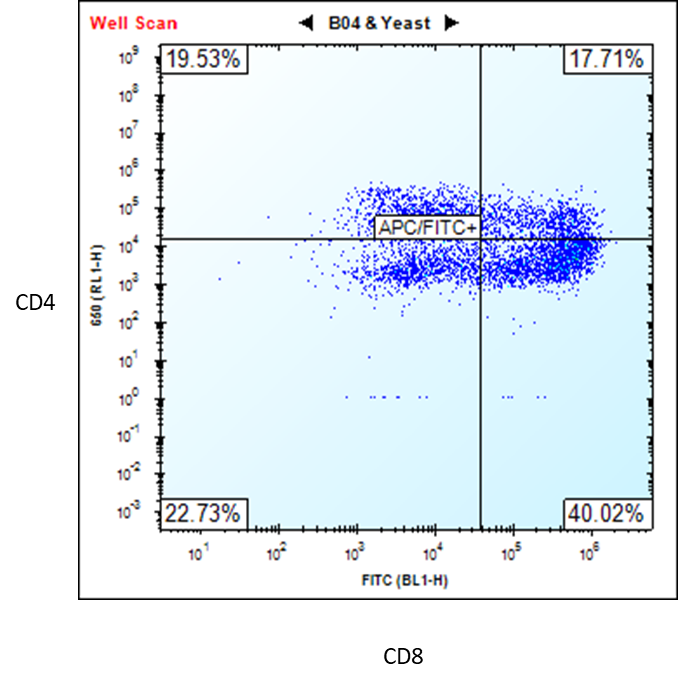
Figure 2. Flow cytometry analysis of ASE-9720 iPSC-derived T cells for T cell biomarkers. The T cells,
differentiated from Applied StemCell’s control iPSC line, ASE-9211, were stained with T cell markers CD4 and
CD8. (Premature T Cells CD4+/CD8+ = 17.71% and Mature T Cells CD8+ = 40.02%) *
*Upon request, the T cells can be purified by FACS or MACS (single positive or double positive). We can also further differentiate the cells to obtain a higher percentage (≥80) of
mature CD8+ T cells.
Case Study 3
Characterization of the ASE-9718 Hematopoietic Progenitor Cells
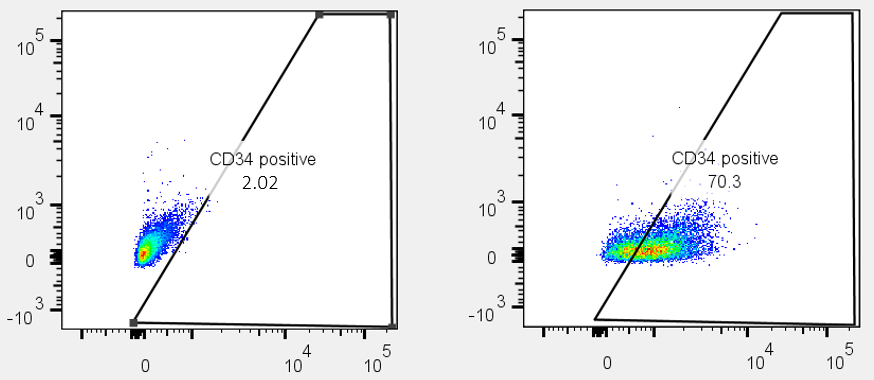
Figure 3. Flow cytometry analysis of ASE-9718 iPSC-derived Hematopoietic Progenitor Cells for an HPC biomarker. Cryopreserved HPCs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in HPC culture basal media. The cells were stained with HPC cell marker, CD34 (right panel) or isotype control (Left panel).
Case Study 4
Characterization of the ASE-9719 Hematopoietic Progenitor Cells
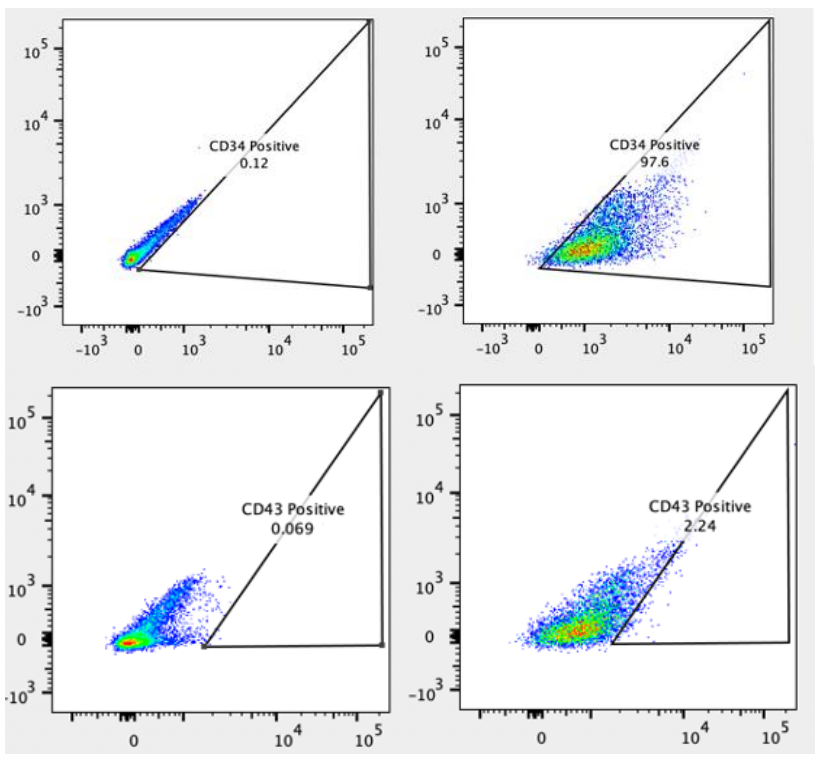
Figure 4. Flow cytometry analysis of ASE-9719 iPSC-derived Hematopoietic Progenitor Cells for HPC biomarkers. Cryopreserved HPCs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in HPC culture basal media. The cells were stained with HPC markers, Top: CD34 (Right panel) or isotype control (Left panel), Bottom: CD43 (Right panel) or isotype control (Left panel).
Application Notes
Applications for iPSC-derived immune cells include:
- Disease modeling for cancer and autoimmune disorders
- iPSC-derived antigen-specific CAR-T/CAR-NK research
- Adoptive transfer and other cell-based therapies
- Antibody discovery
- Drug target discovery and drug screening
- Cell line models for drug toxicity screening
- Cell-based therapies
- Co-culture cells of different lineages to model in vivo heterogeneity of human immune system and cancer pathology
- Multiplexing target attributes
T lymphocytes (T cells): With the FDA approval of two novel T cell-based therapies for treating cancer, Kymriah™ and Yescarta™, the race for immunotherapies for different types of cancer and immune system-based disorders has intensified. These are a type of adoptive cell transfer (ACT) therapies called chimeric antigen receptor-T cell (CAR-T) where a patient’s (autologous) T cells are genetically engineered ex vivo, to recognize and kill tumor-specific antigens expressed on the surface of tumor cells. They leverage the central role played by T lymphocytes in the immune response to treat cancer. Another type of cytotoxic T lymphocyte cell therapy includes directly delivering ex vivo engineered autologous T cell receptors (TCR-T) that recognize tumor-specific intracellular proteins presented as protein fragments by the major histocompatibility complex (MHC) class I proteins.
Although a great success initially in cancer remission and treatment, there are limitations in the technology such as: limited to autologous (patient) T cells, loss of naïve T cells and persistence of long-term immunologic memory as T cells acquire effector functions, and T cell exhaustion due to repeated activation of T cells in cancer patients which severely reduces the amount of cells that can be harvested from the patient. Such limitations may be overcome by using T cells differentiated ex vivo from iPSCs reprogrammed from patient cells. These T cells have rejuvenated T cell characteristics and can be produced in high numbers with consistent and reliable quality. Additionally, iPSCs can be genetically engineering more efficiently than primary T cells and can be used for generating CAR-T and TCR-T cells for immunotherapy applications.
Natural Killer (NK) cells are a type of lymphoid cells that originate from the same progenitor as T cells and B cells. They are an important part of the innate immune response. NK cells are activated in response to interferons or macrophage-derived cytokines. They recognize “non-self” cells without the need for antigen presentation or recognition, executing a rapid immune reaction. The broad cytotoxicity and rapid apoptosis induced by the NK cells help contain virus-infected cells and controlling early signs of cancer while the adaptive immune response is activated to produce cytotoxic T cells to clear the antigens. With the improving success rate of CAR-T cell therapy, scientists are exploring the NK cells to engineer them for CAR-NK cell therapy to target cancer cells. CAR-NK cells have certain advantages over CAR-T cells in that they retain their natural tumor recognition and killing ability; they do not require strict HLA matching and lack the potential to cause graft-versus-host disease (GvHD), they do not have the same safety concerns as seen with CAR-T cells such as cytokine release syndrome, and therefore they can be generated from allogenic donors and possibly developed as off-shelf therapeutic products.
However, purification of NK cells from allogenic hosts is critical since residual T and B cells may cause GvHD and other complications. Primary NK cells are difficult to harvest, purify, and transfect/transduce, and result in a heterogenous population. They are also difficult to standardize due to the heterogeneity in starting material from different donors. As well, the generation of large quantities of highly pure NK cells requires an extended manufacturing process which can compromise recovery of NK cells, their viability, and potency. Therefore, iPSC-derived NK cells offer a consistent and renewable starting material to provide standardizable NK cells without the dependency on donors and complex harvesting and purification processes. They offer a method to generate homogenous populations of CD56+ CD45+ NK cells and subsequently to generate CAR-NK cells for targeted allogenic cancer immunotherapy, even solid tumors.
Morris, E. C., & Stauss, H. J. (2016). Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood, 127(26), 3305-3311.
Kawamoto, H., Masuda, K., Nagano, S., & Maeda, T. (2018). Cloning and expansion of antigen-specific T cells using iPS cell technology: development of “off-the-shelf” T cells for the use in allogeneic transfusion settings. International journal of hematology, 107(3), 271-277.
Minagawa, A., Yoshikawa, T., Yasukawa, M., Hotta, A., Kunitomo, M., Iriguchi, S., ... & Kawai, Y. (2018). Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell stem cell, 23(6), 850-858.
Natural killer cells for cancer immunotherapy: a new CAR is catching up. (). EBioMedicine, 39, 1–2. doi:10.1016/j.ebiom.2019.01.018.
Zeng, J., Tang, S. Y., Toh, L. L., & Wang, S. (). Generation of "Off-the-Shelf" Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells. Stem cell reports, 9(6), 1796–1812. doi:10.1016/j.stemcr.2017.10.020.
FAQs
T Cells: Can the T cells be passaged?
T Cells CD8+: How long can we culture the CD8+ T cells from the frozen vial?
NK Cells: Can the NK cells undergo proliferation?
NK Cells: Can I order the media provided in the iPSC-Derived Human NK Cell Kit (ASE-9708) separately?
NK Cells: What’s the known average cell viability after thawing the cryopreserved NKs?
NK Cells: For how long have these NK cells been expanded before freezing down? (Culture period after iPSC generation)
NK Cells: If these NKs won’t proliferate long enough, how soon will be suitable to do functional assays on that?
HPCs: What is the ability of iPSC-derived hematopoietic progenitor cells to proliferate and differentiate into microglia?
HPCs: Can the HPCs undergo expansion?
HPCs CD34+ : In the step 2.2 : ¨add required hematopoeitic cytokines and growth factors into HPC culture basal media¨ Does that means the media is not ready to use? What type of cytokines and growth factors should I add?
HPCs CD34+: What is the indicated coating for this type of cells? Should I coat the plate even if I use an ultra-low attachment plate?
HPCs CD34+: What is the best plating density?
HPCs CD34+: If I expand the cells can I use tryple for passaging?
HPCs CD34+: How many passages can I do? For how long are they still progenitors?
HPCs CD34+: How often should I change the medium?