Newsletter
Retinal Pigment Epithelium (RPE) Differentiation
Human iPSC-derived retinal pigment epithelium (RPE) provides a physiologically relevant cell line model to understand basic ocular biology and ocular diseases. ASC’s optimized RPE differentiation protocol allows for the generation of high-quality RPE cells that express lineage-committed markers. In just a few weeks (16-20 weeks) you can produce the RPE cells you need to push your research to the next stage.
- RPE-like cells with typical cobblestone morphology and pigmentation
- High-quality and purity cells expressing RPE-specific markers
- RPE differentiated from iPSCs:
- Your healthy/ disease/engineered iPSCs
- iPSC generation from fibroblast/PBMC/ CD34+ cord blood cells
- Optional! Control lines
- Fast turnaround time: 2 months
- GMP iPSC Differentiation Services Available >> Learn More
| RPE | |
| Biomarkers |
RPE65 - Standard |
| MITF - Standard | |
| Z0-1 - Standard | |
| PMEL1 - Inquire |
Looking for iPSCs to genetically modify and further differentiate into RPE? We offer fully customizable iPSC generation from human or non-human tissue samples, and our genome editing experts can use CRISPR or TARGATT™ to genetically alter the iPSCs to suit your projects. If you need to expedite your experimental timeline, ASC also offers off-the-shelf iPSC-derived RPE and Photoreceptor cells. Contact us today to find the best solution for your research.
Products and Services
Case Studies
Case Study 1
Characterization of iPSC-derived ASE-9710 RPE Cells
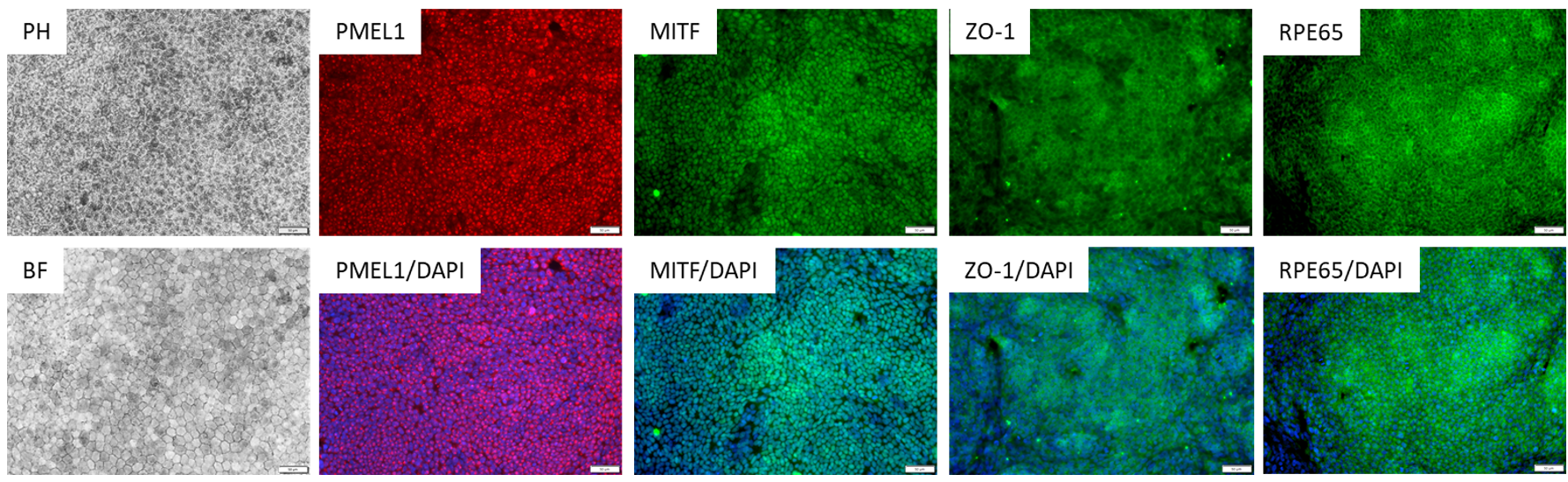
Figure 1. Immunostaining of ASE-9710 iPSC-derived Retinal Pigment Epithelium Cells for retinal pigment
epithelium biomarkers. Cryopreserved RPE cells, differentiated from Applied StemCell’s control iPSC line, ASE9211 were recovered in RPE culture media. The cells were stained with RPE markers, PMEL1, MITF, ZO-1 and
RPE65.
Application Notes
Traditionally, in vitro modeling of retinal pigment epithelium (RPE) cells to understand ocular biology and ocular diseases has used primary RPE from donor tissues or immortalized cell lines. While the primary RPEs exhibit in vivo-like characteristics, they are difficult to source in large quantities from the same donor. On the other hand, the immortalized cell lines like hTERT-RPE have overcome sourcing issues but do not show the same gene expression or functional characteristics of in vivo RPEs are not truly representative.
iPSC-derived RPEs represent the best of both worlds: as primary cells, they show in vivo-like genetic and functional characteristics and with the ability to generate large quantities of isogenic cell lines from the same parental iPSC line. In fact, transplantation of autologous iPSC-derived RPEs have been successful in rescuing vision in animal models and are being tested in patients in one of the first clinical trials in regenerative medicine.
Applications:
- Disease modeling such as age-related macular degeneration (AMD), diabetic retinopathy
- Preclinical regenerative medicine research (adoptive transfer)
- Drug testing and toxicity screening
- High throughput drug target discovery