Newsletter
Immune Checkpoint Mouse Models
Worldwide shipping!
Applied StemCell’s off-shelf mouse catalog offers >100 humanized immune checkpoint mouse models, with single, double, and triple humanized gene models.
- Knock-in of humanized counterparts of immune checkpoint gene into the endogenous locus.
- Proprietary human transgene knock-in strategy to enable screening of many different antibodies and immuno-therapy compounds.
- In vivo and in vitro validated for physiological expression of human version of the genes (in-house and through collaborators).
- Fully functional murine immune system
- The humanized immune checkpoint mice have been generated in the most widely used C57BL/6J background used in immunology and oncology studies.
- Ideal for immunology and antibody/ immuno-oncology therapy efficacy studies and drug discovery research.
_____________________________________________________________________________________________________________________
Antibody Efficacy Testing Service
Leverage ASCs cost-effective evaluation of in vivo efficacy and fast timelines to screen your early-stage lead antibodies using our antibody efficacy testing platform:
Workflow includes:
- Project Proposal: details on the test antibody (and positive and negative control), project design, scope of the project
- Receive antibody(ies) from client
- Tumor cell implantation and development in humanized immune checkpoint mouse models (mean tumor size reaches 100-150 mm^3)
- Administration of antibodies to humanized immune checkpoint mouse tumor models
- In vivo evaluation of antibody efficacy: tumor size and body weight, twice a week for 3-4 weeks
- Final report with data and figures
- Report will include comprehensive information about experimental/ project outline, project schedule, survey notes, raw data for efficacy parameters measured/ endpoints, calculated/ integrated results, raw and integrated figures (graphs and other figures corresponding to endpoint measurements).
* Endpoint: Tumor burden should not exceed 10% of the animal’s bodyweight. The study will be terminated with euthanizing all animals when the mean tumor volume reaches about 3000 mm^3.
Optional post-mortem tests include: Blood RT/Biochemical analysis; FACS analysis on PBMC (including CBA); serum/ plasma preparation and then ELISA, MSD or WB analysis, qPCR,WB, IHC, IF, TILs analysis
Timeline: 2-3 months after receipt of antibodies
LEARN MORE
Products and Services
Technical Details
Immunotherapy has revolutionized the treatment for cancer by boosting the patient’s own immune system to attack cancer cells. This has been very efficacious in treating many different types of cancer either as a standalone treatment option or in combination with other types of cancer treatment and can be largely attributed to immune checkpoint blockade.
The immune response to an antigen is regulated by a balance between stimulatory and inhibitory signals through a signal transduction pathway of receptors and ligands called Immune checkpoints. Tumor cells hijack these checkpoint pathways by either overexpressing inhibitory immune checkpoints or the suppression of stimulatory immune regulators. These immune checkpoints are the target of a rapidly growing area of immuno-oncology which aims to and train the immune system to recognize and eliminate cancerous cells.
To support the development of these drugs through the clinical stages, preclinical animal models that offer efficient translation of evaluation studies are needed in order to assess the efficacy and safety of these novel therapies. Mouse model are the most commonly and widely accepted in vivo models for preclinical studies. However, the differences in the humans and mouse immune systems with only a 60% homology results in discrepancy in drug performance between preclinical and clinical studies. A preclinical model that mimics the human immune system and cancer development could provide the necessary tumor environment to evaluate the efficacy of these drugs.
To the bridge the gap in bench-to-bedside translation, Applied StemCell has developed 100+ off-shelf, mouse models with knock-in of the human ortholog of immune checkpoint and immunomodulatory genes to replace the entire or a part of the endogenous mouse gene. These humanized knock-in immune checkpoint mouse models offer an ideal platform for developing tumor mouse models for basic research and drug testing.
- Single, double, triple humanized gene knock-in to enable study of combination immuno-oncology therapies
- Knock-in of humanized counterparts of immune checkpoint gene into the endogenous locus.
- Proprietary knock-in strategies in the widely used C57BL/6J background to utilize the complexities of the murine immune system along with the humanized gene expression.
- Can be combined with CDX and PDX tumor engrafting to develop clinically relevant tumor models.
- In vivo and in vitro validated for physiological expression of human version of the genes (in-house and through collaborators).
- Fully functional murine immune system
These animal models have been validated using tumor engrafts and standard-of-care immunotherapy drugs, and have been successfully used to test the efficacy of novel immunotherapy candidate drugs in early-stage screening for clients before they need expensive GLP studies.
These humanized immune system mouse models are ideal for immunotherapy efficacy studies and drug discovery research for cancer and other immune diseases.
Don’t see a particular mouse model in our repository? We can custom engineer humanized gene knock-in mouse models using our 11+ years of expertise in genetically engineered mouse model (GEMM) generation using CRISPR/Cas9.
Why Choose Applied StemCell?
- 11+ years of expertise in mouse model genetic engineering
- Most up-to-date CRISPR protocols for efficiency and affordability
- Germline transmission included
- AAALAC-accreditation and SPF facilities
- We ship globally
- We also offer downstream validation of your models and drug screening services available
Application Notes
Validation Data for Humanized PD-L1 Mouse
Strain Name: C57BL/6J-Cd274em1(hPD-L1)/Asc Strain Background: C57BL/6J
Programmed cell death ligand-1 (PD-L1) is a critical immune checkpoint molecule targeted for immunotherapies. This protein, also known as the cluster of differentiation 274 (CD274) or B7 homolog 1 (B7H1), is a transmembrane protein encoded by the CD274 gene and is the ligand for the PD-1 cell surface receptor. The binding of PD-L1 to PD-1 expressed on activated T cells transmits an inhibitory signal that inactivates cytotoxic T cells. This inhibitory mechanism is leveraged by tumor cells which overexpress PD-L1 and thereby inhibit the function of tumor-infiltrating T cells in order to escape immune surveillance. The humanized PD-L1 mouse model enables better translational results to test the efficacy of anti-PD-L1 immunotherapies.
Construction Strategy
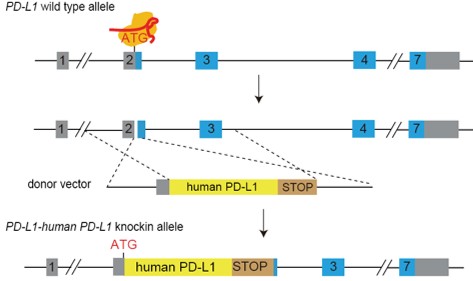
Figure 1. Generation strategy of humanized PD-L1 mice. The full-length protein coding sequence for human PD-L1 was placed immediately downstream of the start codon of the mouse endogenous Pd-l1 gene, followed by a poly(A) site, so that the expression of endogenous Pd-l1 in the mouse was replaced by the expression of fully humanized PD-L1 protein. This mouse model was generated in the C57BL/6J genetic background.
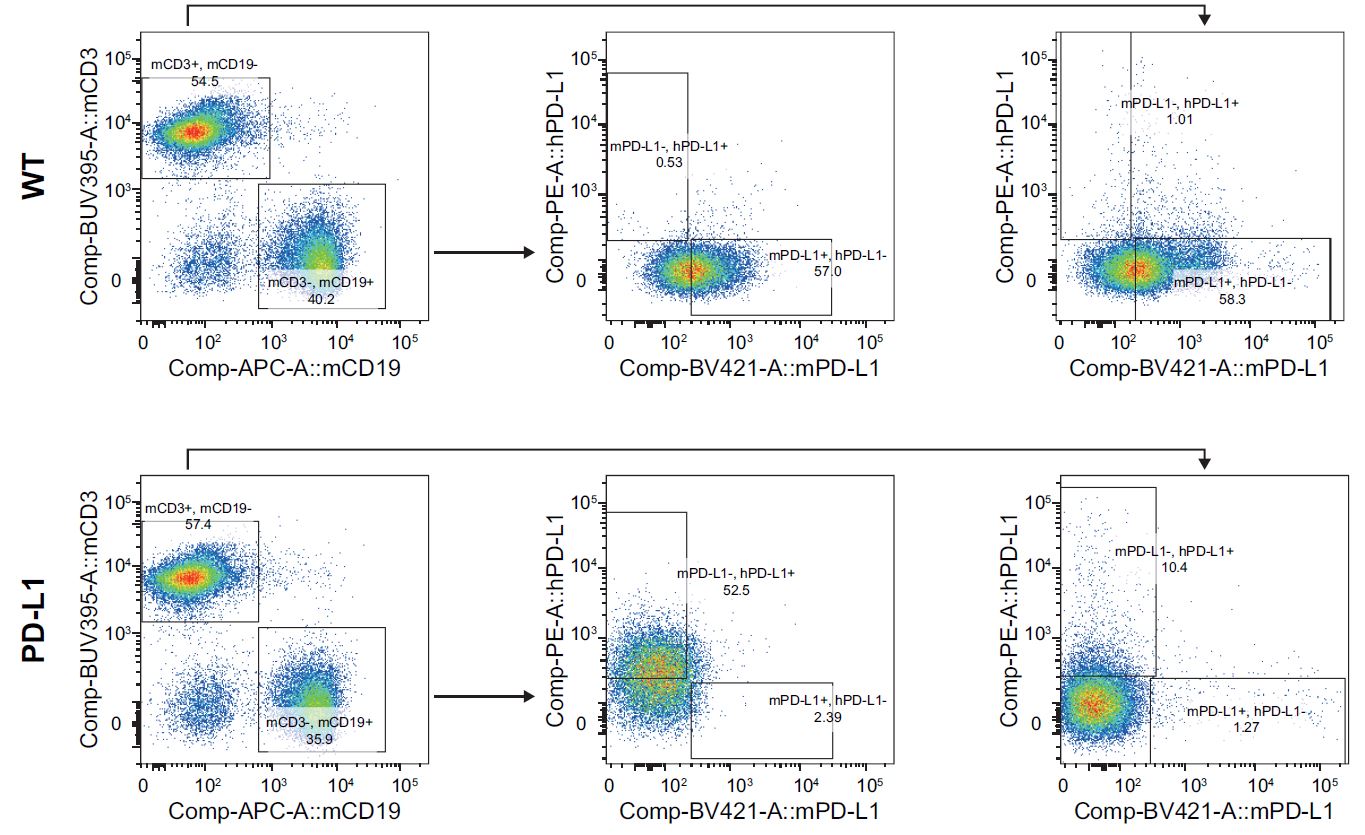
Figure 2. hPD-L1 expression in spleen T cells after stimulation. Flow cytometry analysis of spleen lymphocytes collected from homozygous humanized PD-L1 mice and wild-type C57BL/6J mice. The results showed that the expression of human PD-L1 can be detected in both T cells and B cells collected from the spleen of homozygous humanized PD-L1 mice (completed in collaboration with partners).
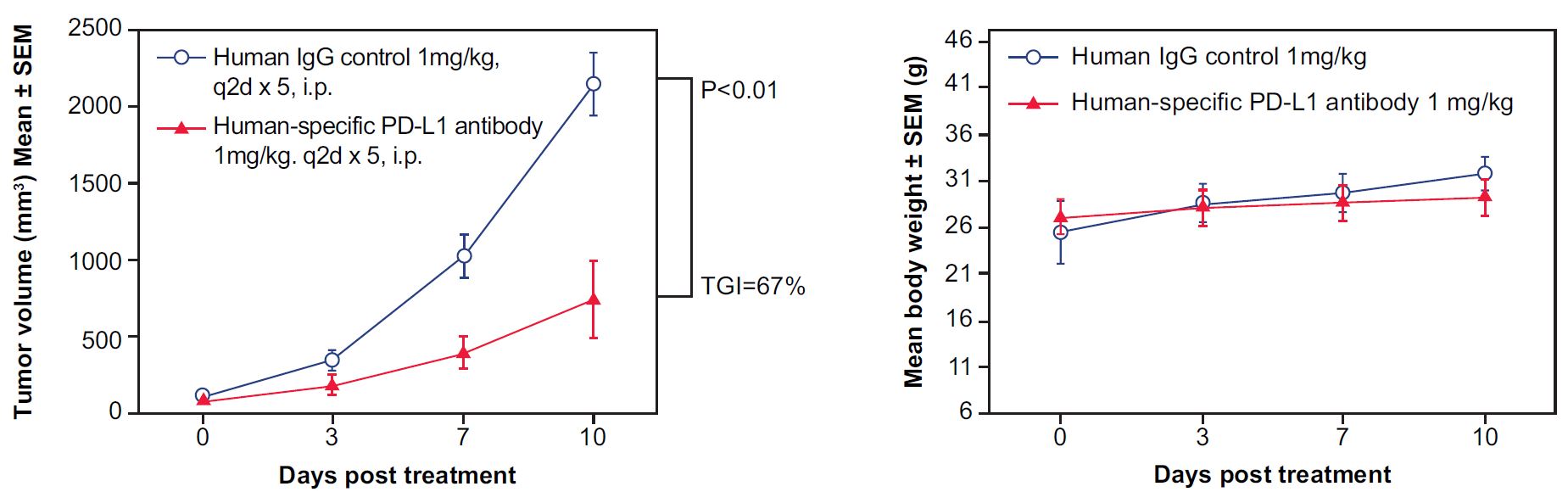
Figure 3. In vivo validation of anti-tumor efficacy in a MC38 tumor-bearing model of humanized PD-L1 mice. Mouse colon cancer cells MC38 engineered to express human PD-L1 were implanted subcutaneously into homozygous, humanized PD-L1 mice. The mice were randomly assigned into two groups when the tumor volume reached 100 mm3, with one group receiving human IgG as a control and the other receiving a human-specific, PD-L1 antibody (n=5). The human PD-L1 blocking antibody significantly inhibited tumor growth in the homozygous humanized mice (Left) without affecting overall body weight (Right), suggesting that the humanized PD-L1 mice represent an ideal model for evaluating the efficacy of therapeutic antibodies targeting human PD-L1. (TGI: tumor growth inhibition; 67%; p < 0.001), demonstrating that the humanized PD-L1 mice are a good in vivo model for validating the efficacy of antibodies targeting human PD-L1.
FAQs
Does the humanized PD-1/PD-L1 Dual Immune Checkpoint Knock-in Mice express the full length humanized gene or a chimera with humanized extracellular domain and mouse mPd-1 and mPd-L1 intracellular domain?



