Newsletter
Human ACE2 Mice for COVID-19 Research
Humanized ACE2 Mouse Models Available Now
To aid the global research efforts to find a solution to the COVID-19 pandemic, Applied StemCell has leveraged its site-specific TARGATT™ knockin technology to engineer two fully humanized ACE2 (hACE2) mouse models with lung epithelium-specific expression driven by the K18 promoter. These TARGATT™ hACE2 mouse models have the K18-hACE2 transgene integrated into a preselected, well-characterized safe harbor locus (genomic hotspots): mH11 or mRosa26 and might be more representative to model the severity of the SARS-CoV-2 infection. The TARGATT™ hACE2 mouse models are ideal to help understand the severe pathogenesis of COVID-19, and to evaluate the efficacy of antiviral drugs and vaccines.
Products and Services
Technical Details
Applied StemCell has two complementary technologies, CRISPR/Cas9 and TARGATT™ that can aid in the development of humanized ACE2 mouse models under the control of any promoter of choice. We can also engineer these mouse models as conditional or inducible expression models for better control of the humanized ACE2 expression.
Building on our mouse genetic engineering expertise and our TARGATT™ site-specific gene integration technology, we have developed two humanized ACE2 mouse models that expresses the hACE2 under the control of the lung epithelium-specific promoter, K18. The K18-hACE2 transgene has been integrated into a well-characterized, safe harbor locus (mH11 or mRosa16) to enable strong, consistent expression of the humanized gene. The site-specific insertion of the transgene overcomes challenges posed by random integration such as disruption of mouse internal genes and gene silencing. We predict this model will recapitulate the SARS-CoV-2 infection in humans better than the currently available models.
Advantages of ASC’s TARGATT™ Humanized ACE2 (hACE2) mouse model for COVID-19 research:
- Full length human ACE2 gene
- Lung alveolar epithelium-specific hACE2 expression driven by K18 promoter (makes the mice more susceptible to the virus)
- Site-specific integration into a well characterized safe harbor locus: Rosa26 or H11
- Avoids problems associated with random integration such as gene silencing, disruption of endogenous genes
- Engineered in the widely used and well-characterized C57BL/6J mouse strain
- Mouse models can be shipped worldwide
In addition, please browse through our repository for other research-ready ACE2 mouse models.
|
|
Catalog # |
Genetic Modification |
Modification Method |
Modification locus |
Promoter |
Mouse Strain |
Availability |
|
Humanized ACE2 Mouse Model – H11/mACE Knockout |
580-6 |
Knockin/Knockout |
TARGATT™ |
mHipp11 (safe harbor) |
K18 |
C57BL/6 |
Available now |
|
Model Characteristics - Human ACE2 gene driven by the epithelial-specific K18 promoter, inserted at a genomic “hotspot” (H11 locus) to enable a higher expression in the lungs. This could increase susceptibility of the hACE2 mice to SARS-CoV-2 infection. - mACE has been knocked out. |
|||||||
|
Humanized ACE2 – H11 |
580-1 |
Knockin |
TARGATT™ |
mHipp11 (safe harbor) |
K18 |
C57BL/6 |
Available now |
|
Model Characteristics Human ACE2 gene driven by the epithelial-specific K18 promoter, inserted at a genomic “hotspot” (H11 locus) to enable a higher expression in the lungs. This could increase susceptibility of the hACE2 mice to SARS-CoV-2 infection. |
|||||||
|
Humanized ACE2 – Rosa26 |
580-2 |
Knockin |
TARGATT™ |
mRosa26 (safe harbor) |
K18 |
C57BL/6 |
Available now |
|
Model Characteristics Human ACE2 gene driven by the epithelial-specific K18 promoter, inserted at a genomic “hotspot” (Rosa26 locus) to enable a higher expression in the lungs. This could increase susceptibility of the hACE2 mice to SARS-CoV-2 infection. |
|||||||
|
Humanized ACE2 |
ASHU-200218 |
Knockin |
CRISPR/Cas9 |
mACE2 |
mAce2 |
C57BL/6 |
Available now |
|
Model Characteristics A human ACE2-flag-Wpre-pA expression cassette was inserted at the mAce2 start codon site. |
|||||||
|
Ace2-(2A-CreERT2) |
ASKI-200187 |
Knockin |
CRISPR |
mACE2 |
mACE2 |
C57BL/6 |
Available now |
|
Model Characteristics A 2A-CreERT2-WPRE-polyA expression cassette was knocked into the 3’ end of the mouse Ace2 gene. |
|||||||
|
ACE2-KO |
ASCKO-190906 |
Knockout (deletion of exons 3-7) |
CRISPR/Cas9 |
mAce2 |
mAce2 |
C57BL/6 |
Available now |
|
Model Characteristics Expresses a truncated protein. |
|||||||
Don’t see a particular COVID-19 mouse model in our repository? We can custom engineer a mouse model-of-choice using our 12+ years of expertise in genetically engineered mouse model (GEMM) generation using the CRISPR/Cas9 and our proprietary TARGATT™ technologies.
1. "Novel coronavirus structure reveals targets for vaccines and treatments". National Institutes of Health (NIH). 2 March 2020. Archived from the original on 1 April 2020. Retrieved 3 April 2020.
2. Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., ... & Müller, M. A. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell.
3. Shang, J., Ye, G., Shi, K., Wan, Y., Luo, C., Aihara, H., ... & Li, F. (2020). Structural basis of receptor recognition by SARS-CoV-2. Nature, 581(7807), 221-224.
4. Glass, W. G., Subbarao, K., Murphy, B., & Murphy, P. M. (2004). Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. The Journal of Immunology, 173(6), 4030-4039.
5. Li, W., Greenough, T. C., Moore, M. J., Vasilieva, N., Somasundaran, M., Sullivan, J. L., ... & Choe, H. (2004). Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. Journal of virology, 78(20), 11429-11433.
6. Chow, Y. H., O’Brodovich, H., Plumb, J., Wen, Y., Sohn, K. J., Lu, Z., ... & Buchwald, M. (1997). Development of an epithelium-specific expression cassette with human DNA regulatory elements for transgene expression in lung airways. Proceedings of the National Academy of Sciences, 94(26), 14695-14700.
7. McCray, P. B., Pewe, L., Wohlford-Lenane, C., Hickey, M., Manzel, L., Shi, L., ... & Meyerholz, D. K. (2007). Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of virology, 81(2), 813-821.
8. Tseng, C. T. K., Huang, C., Newman, P., Wang, N., Narayanan, K., Watts, D. M., ... & Peters, C. J. (2007). Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. Journal of virology, 81(3), 1162-1173.
9. Yang, X. H., Deng, W., Tong, Z., Liu, Y. X., Zhang, L. F., Zhu, H., ... & Xu, Y. F. (2007). Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comparative medicine, 57(5), 450-459.
10. Sutton, T. C., & Subbarao, K. (2015). Development of animal models against emerging coronaviruses: From SARS to MERS coronavirus. Virology, 479, 247-258.
11. Bao, L., Deng, W., Huang, B., Gao, H., Liu, J., Ren, L., ... & Qu, Y. (2020). The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. BioRxiv.
12. Sun, S. H., Chen, Q., Gu, H. J., Yang, G., Wang, Y. X., Huang, X. Y., ... & Guo, Y. (2020). A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host & Microbe.
13. Cockrell, A. S., Leist, S. R., Douglas, M. G., & Baric, R. S. (2018). Modeling pathogenesis of emergent and pre-emergent human coronaviruses in mice. Mammalian Genome, 29(7-8), 367-383.
Case Studies
Case Study 1
ACE2 Mouse Model Characterization: PCR Based mRNA Expression
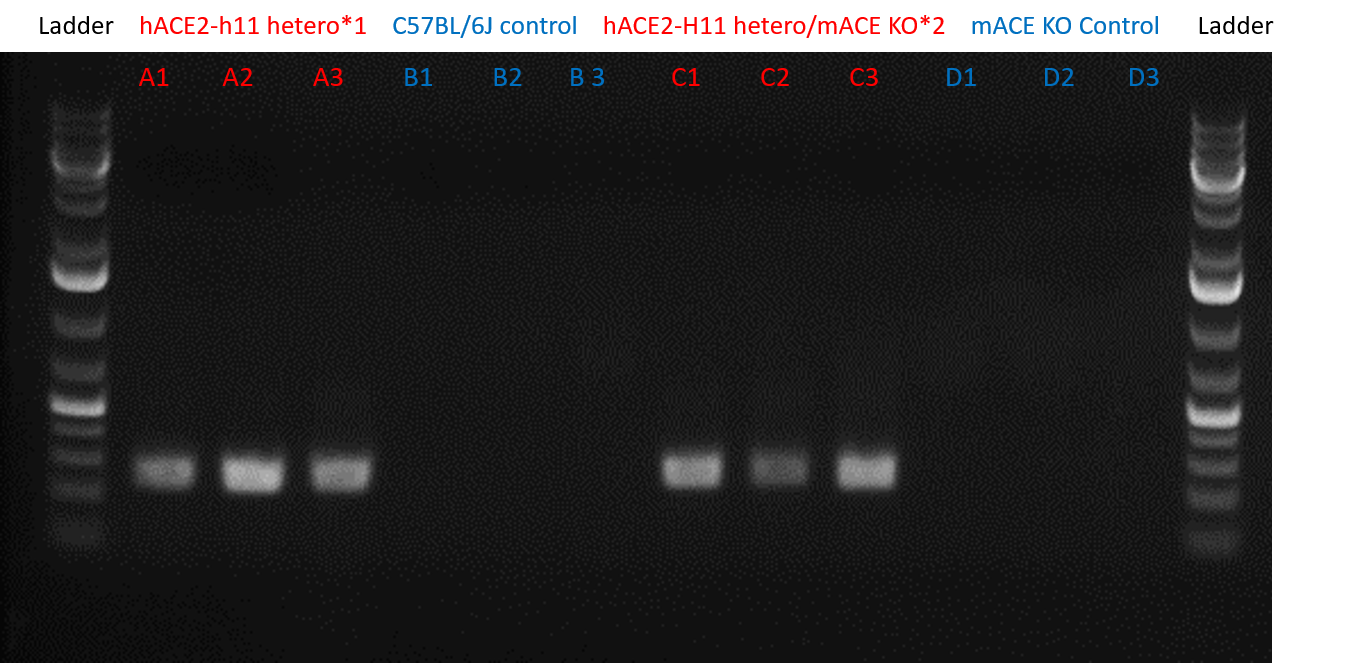
Figure 1: mRNA PCR Gel Confirming hACE2 Expression. The ~250bp product in lanes A1-A3 and C1-C3 confirmed hACE2 expression of the *1 TARGATT™ Humanized ACE2 Mouse Model (H11 Locus), #580-1 and *2 TARGATT™ Humanized ACE2 Mouse Model, respectively. The two controls, C57BL/6J and mACE KO did not present a band at around ~250bp.
Application Notes
The coronavirus disease 2019 (COVID-19) is a viral infection that was declared a pandemic on March 11, 2020. The virus responsible for this pandemic is the severe acquired respiratory syndrome coronavirus 2 (SARS-CoV-2, also called 2019-nCoV, HCoV-19, hCoV-19), a novel virus in the Coronaviridae family of viruses.
The SARS-Cov-2 is a positive strand RNA virus that is said to have zoonotic origins. It invades the epithelial cells lining mucosal surfaces (such as the lungs and intestine) through the binding of the viral spike (S) protein layer (that forms the characteristic the “corona” of the virus) to the angiotensin converting enzyme 2 (ACE2) receptor and causes acute respiratory illness and infection. The SARS-CoV-2 virus though closely related to the first SARS virus strain, SARS-CoV which caused a global outbreak in 2003, binds to ACE2 with a much higher affinity than the original strain 1,2,3.
Understanding the pathology of the disease is crucial to developing antiviral therapy and vaccines to treat and prevent infection. Animal models that mimic the human course of the disease are very important for thorough testing of the developed therapies before approval for use in patients. Models that effectively mimic the tropism of the virus in the nasal mucosa and intestine, viral shedding, and the pathology of the severe respiratory illness will be able to successful in evaluating therapies and vaccines.
Research with mouse models of the first severe acquired respiratory syndrome revealed that the murine ACE2 (mACE2) was not as susceptible to the virus infection as the human ACE2 (hACE2) 4,5. Transgenic mouse models were then generated to express the hACE2 under different constitutive promoters such as the human cytokeratin 18 (K18) promoter (specific to airway, gastrointestinal, liver and kidney epithelium) 6,7, chicken beta-actin promotor with a cytomegalovirus IE enhancer (CAG) 8 and the murine ACE2 promoter 9. These mouse models were generated by random transgene insertion at an unspecified locus and both mACE2 and hACE2 were expressed together. In all these models, hACE2 was expressed in high levels in the lungs, intestinal epithelia, liver, colon, and kidney, and when challenged with a clinical strain of the SARS-CoV virus, the mice developed severe infections along with associated pathological findings that correlated with level of hACE2 expression 10.
However, in mice with mACE2 promoter driven expression of the hACE2, the mice showed less severe clinical illness and mortality for both the SARS-CoV and the SARS-CoV-2 11 viruses, that may be due to the lower expression of hACE2 driven by the mACE2 promoter 9,10.
Recently, authors Sun and others generated a humanized ACE2 transgenic mouse model using CRISPR/Cas9, to study the SARS-CoV2. They inserted the full length hACE2 sequence into the endogenous murine ACE2 locus, along with a downstream TdTomato reporter gene. While the previous transgenic models retained the mACE2 expression along with hACE2 expression, in this model the mACE2 expression was disrupted and only the hACE2 was expressed 12. The hACE2-TdTomato transgenic mice did not show any clinical signs of infection or mortality and all the infected mice recovered. The pathological outcomes however recapitulated those from the COVID-19 patients thereby establishing another mouse model for a milder version of COVID-19 13.
Applied StemCell’s TARGATT™ hACE2 mouse models with the K18-hACE2 transgene integrated into mH11 or mRosa26 locus offer the advantage of site-specificity and tissue-specific expression to model the severe pathogenesis of the SARS-CoV-2 infection. Please inquire for details.
FAQs
580-6 & 580-1: Are the mice models homozygous or heterozygous?



