Newsletter
iPSC Differentiated Cardiomyocytes
Ready-to-use, beating human cardiomyocytes differentiated from normal iPSCs (ASE-9211) are now available for purchase. The high-purity (≥80%) cardiomyocytes beat 2-3 days after recovery and express high levels of cnTnT. They are perfect in vitro tools for electrophysiology and biochemical assays, preclinical cardiac safety assessment, drug development, toxicity screening, and genetic studies.
- High-purity (≥80%)
- Integration-Free Differentiation Protocol
- Derived from our well-characterized, NIST iPSC Control Line
- GMP iPSC Products & Services >> Learn More
If you are looking to generate your own cardiomyocytes, ASC offers custom iPSC cardiomyocyte differentiation services. We can even assist in the development of any assay you may require for your research.
![]() Beating iPSC-Derived Cardiomyocytes - Video 1| Applied StemCell, Inc.
Beating iPSC-Derived Cardiomyocytes - Video 1| Applied StemCell, Inc.
![]() Beating iPSC-Derived Cardiomyocytes - Video 2 | Applied StemCell, Inc.
Beating iPSC-Derived Cardiomyocytes - Video 2 | Applied StemCell, Inc.
Products and Services
Case Studies
Case Study 1:
Characterization of the Cardiomyocytes Provided in the ASE-9703 Kit
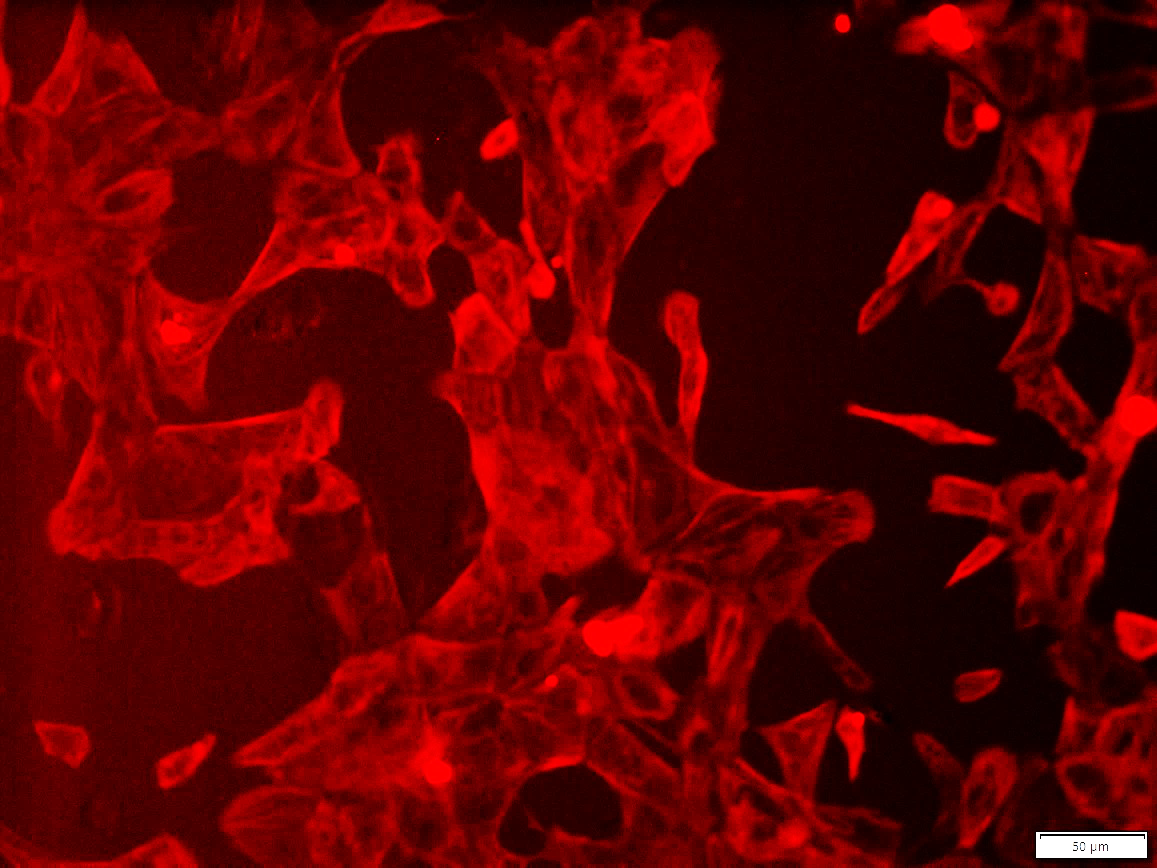
Figure 1. Immunostaining of ASE-9703 iPSC-derived cardiomyocytes for a cardiomyocyte biomarker.
Cryopreserved cardiomyocytes, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were
recovered in cardiomyocyte culture media. The cells were stained with cardiomyocyte marker cTnT (red) on day
2 after recovery. Image at 20x magnification.
Case Study 2:
Applied StemCell, Inc. has extensive expertise with iPSC cardiomyocyte differentiation. Previously, one of ASC's projects resulted in the development of high purity (>90%), beating human iPSC-derived Cardiomyocytes (Video 1) they expressed typical markers of cardiomyocytes, e.g. TNNT/cTnT and α-Actinin (Figure 1) and were validated for functional viability by patch-clamp and Fluo-4TM Direct Calcium Assay (Video 2). ASC’s cardiomyocytes were used for electrophysiological and biochemical assays, for example, the effect of compounds on the heart rate can be assayed by impedance-based measurements (Figure 2).
Characterization of iPSC-differentiated Cardiomyocytes
Video 1: Beating iPSC-derived Cardiomyocytes
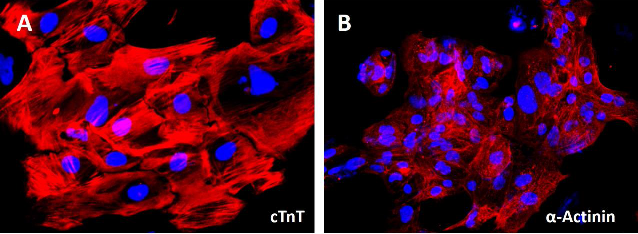
Figure 2. After replating in Cardiomyocyte Maintenance Medium on the Matrigel-coated plates for 2 days, more than 85% of the iPSC-derived cardiomyocytes express cTnT and α-Actinin (Figure A and B).
Video 2: Calcium Flux of iPSC-derived Cardiomyocytes
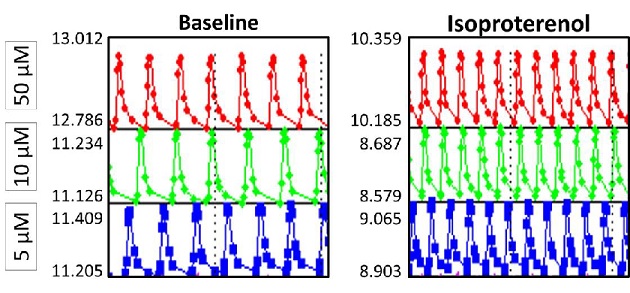
Figure 3. Beating rate of iPSC-derived cardiomyocytes measured with xCELLigence RTCA Cardio System (ACEA Biosciences and Roche Applied Science). Cells were treated with various dosages of Isoproterenol. (Left) Before Treatment (50 μM; 10 μM; 5 μM); (Right) 30 min after treatment (50 μM; 10 μM; 5 μM).
Publications
iPSC-differentiated cell lines
- Kussauer, S., David, R., & Lemcke, H. (2019). hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro-Electrode-Array Analyses Can Tell Us. Cells, 8(11), 1331.
- Cheng, F., Fransson, L. Å., & Mani, K. (2019). The cyanobacterial neurotoxin β-N-methylamino-l-alanine prevents addition of heparan sulfate to glypican-1 and increases processing of amyloid precursor protein in dividing neuronal cells. Experimental Cell Research. https://doi.org/10.1016/j.yexcr.2019.03.041
- Daily, N. J., et al. (2017). High-Throughput Phenotyping of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Neurons Using Electric Field Stimulation and High-Speed Fluorescence Imaging. ASSAY and Drug Development Technologies. 15(4): 178-188. https://doi.org/10.1089/adt.2017.781
- Daily, N. J., Santos, R., Vecchi, J., Kemanli, P., & Wakatsuki, T. (2017). Calcium transient assays for compound screening with human iPSC-derived cardiomyocytes: Evaluating new tools. Journal of evolving stem cell research, 1(2), 1.
- Daily, N. J., et al. (2015). Journal of Bioengineering & Biomedical Science, 2015.
- Daily, N. J., et al. (2017). High-Throughput Phenotyping of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Neurons Using Electric Field Stimulation and High-Speed Fluorescence Imaging. ASSAY and Drug Development Technologies. 15(4): 178-188. https://doi.org/10.1089/adt.2017.781
- Daily, N. J., Santos, R., Vecchi, J., Kemanli, P., & Wakatsuki, T. (2017). Calcium transient assays for compound screening with human iPSC-derived cardiomyocytes: Evaluating new tools. Journal of evolving stem cell research, 1(2), 1.
- Daily, N. J., et al. (2015). Journal of Bioengineering & Biomedical Science, 2015.