Newsletter
Knockout Mouse Model Generation
Generate your gene knockout mouse model for inactivating genes either by frameshift mutation or by fragment excision, using CRISPR/Cas9. Gene knockout or disruption in mouse models provides an in vivo environment to study the function or role of the gene of interest. Applied StemCell has optimized and most up-to-date gene targeting strategies to generate the knockout mouse model of your choice. We can generate constitutive knockout mice, or for a more advanced study of the gene function, conditional/ inducible knockout models in as little as 5 months (F1 germline transmitted).
- Affordable! Low prices and founder guarantee
- F1 breeding for germline transmission
- Novel CRISPR strategy for higher efficiency and success rate
- Animal IP belongs to customers
- Fast turnaround
- Free consultation
- Downstream assay services - available
- We also offer a repository of off-shelf mouse models
Other Available Mouse Generation Service
Products and Services
Case Studies
Case Study 1
CRISPR Knock-out Model - Generation of a site-specific ~ 600 bp CRISPR knock-out mouse model
Goal: To delete a 587 bp fragment from a specific site in C57Bl/6 mouse genome using CRISPR
To achieve the model, three procedures were implemented. In the first step, a mixture of active guide RNA molecules (gRNAs) and qualified Cas-9 mRNA was injected into the cytoplasm of C57BL/6 embryo. The second step was to screen new mice born from the microinjection using PCR. And the third step was to confirm the positive animals by sequencing the modified region in the desired mouse locus.
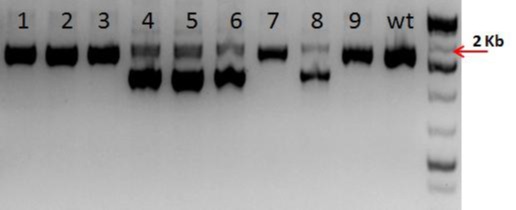
Figure: Mice# 4, 5, 6, 8 were identified as F1 germline transmitted animals carrying the ~ 600 bp deletion. The lower band was extracted, purified and sequenced. The sequence results showed that the deletion removed the entire exon and was identical to parental sequence (not shown).
Case Study 2
CRISPR Knock-out Model -Deletion of a ~100 bp fragment from exon of gene of interest
Goal: To delete a ~100 bp fragment from the N-terminus of the gene of interest in C57BL/6 mouse genome using CRISPR.
The mouse model required a ~ 100 bp DNA fragment to be deleted from the N-terminal of the gene of interest in order to cause a frame shift in downstream gene sequence, thereby leading to loss of protein. For this, we followed a well optimized protocol involving 3 steps: (1) a mixture containing active guide RNA molecules (gRNA) and qualified Cas-9 mRNA was injected into C57BL/6 (B6) embryos; (2) new mice born from the microinjection were screened by PCR; (3) potential positive animals were confirmed for the accurate sequence alterations by sequencing purified PCR products.
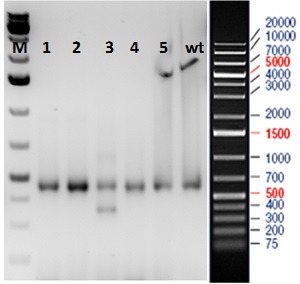
Figure: PCR amplification of exon region of F0 mice. Five mice born from the embryos microinjected with CRISPR cocktail were subjected to PCR amplification using genotyping primers. Mouse#3 was shown to carry a deletion fragment which was purified and sequenced.
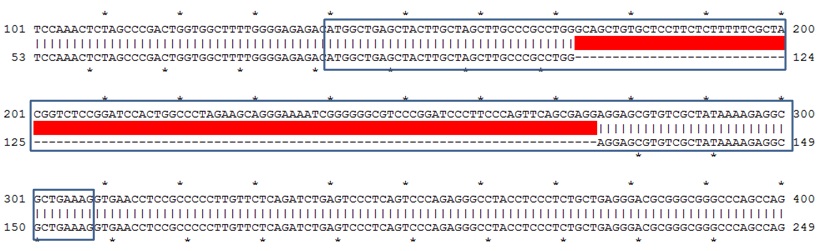
Figure: Sequence alignment of wild type B6 mouse and founder mouse #3 showing deletion of the exon in the coding region(dark frame).



