Newsletter
Mesenchymal Stem Cell (MSC) Services
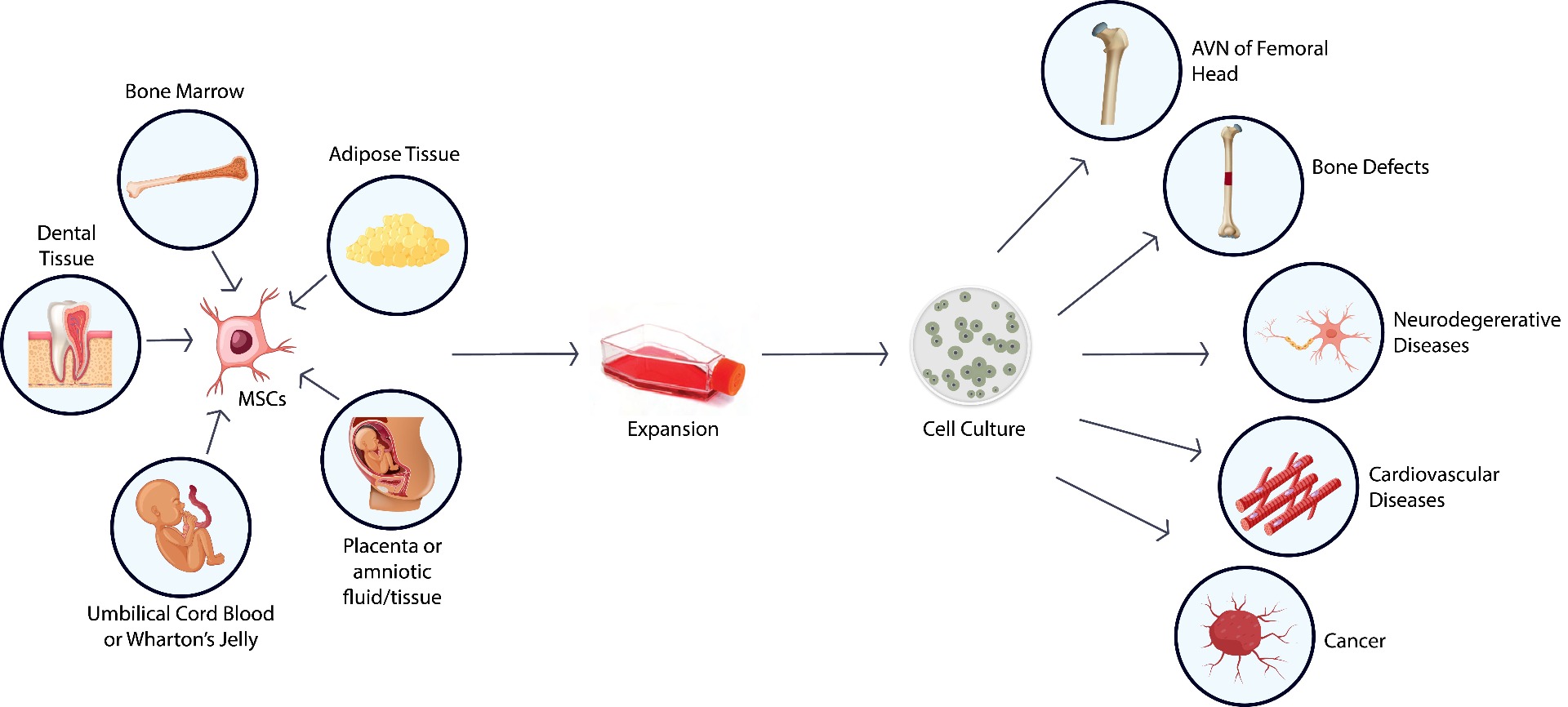
Mesenchymal stem cells (MSCs) have the capacity of multi-direction differentiation making them an ideal cell therapy starting material. At ASC, we offer end-to-end stem cell solutions, including cell isolation, expansion and banking, characterization, and differentiation.
End-to-end MSC solutions for cell therapy manufacturing
- MSC Isolation
- From umbilical cord blood and stroma, adipose tissue, placenta, bone marrow & dental tissues
- Expansion & Banking Services
- Banking; ~2.5 Weeks
- Cell Expansion; ~ 2 Weeks
- 2D and 3D manufacturing
- Characterization Service
- Antigen expression (CD73+, CD90+, CD105+, CD34-, CD45-, CD11b-, CD14-, CD19-, CD79a-, HLA-, DR-)
- Adherence to plastic, Tri-Lineage Differentiation, Immunomodulation, Karyotyping, STR Analysis, Customization options are available
- Differentiation Service
- Customer-provided protocol differentiation with facilitated tech transfer
MSC Isolation, Expansion & Banking Service
- MSCs isolation from adipose tissue, bone marrow, dental tissues, umbilical cord blood and stroma, placenta
- MSC isolation and expansions processed in GMP cleanrooms
- GMP production and documentation available for IND application and clinical trials
MSC Expansion Technology
| Hyper Flasks | Hyper Stack-12 | Hyper Stack-36 | Cell Factory |
|
175cm2 x 10 layers: |
500cm2 x 12 layers: |
500cm2 x 36 layers: |
632cm2 x 40 layers: |
MSC Characterization
- Antigen expression
- (CD73+, CD90+, CD105+, CD34-, CD45-, CD11b-, CD14-, CD19-, CD79a-, HLA-, DR-)
- Adherence to plastic surface
- Tri-Lineage Differentiation
- Immunomodulation
- Karyotyping
- STR Analysis
- Customization options are available
MSC Differentiation
- Differentiation requires expert monitoring and handling
- We can carry out the differentiation of your MSCs using your protocols in a safe controlled environment
- Facilitated tech transfer
- Detailed project updates are provided throughout the experimental project
- Custom Osteogenic Lineage & Chondrogenic Lineage Differentiation Services – Available
Products and Services
Application Notes
MSC Therapeutic Application
ASC Or Customer SOP
- Technology transfer
- SOP establishment
- cGMP control
- Documentation for clinical pre-IND filing
![]()
Support Materials
GMP Facility In Milpitas, California
Total space of 15,000 sq. ft. & 10,000 sq. ft. of lab space – 5 Cleanrooms
- Quality ISO 13495 certified; guidelines established following Title 21CFR (Code of Federal Regulations) Part 1271 (Current Good Manufacturing for Cells, Tissues, and Cellular and Tissue-Based Products)
- Facility includes:
- GMP suite of ~2000 sq. ft. Class 10,000 cleanroom, with HEPA filters, airflow, interlock passing-through window, gowning room, etc. that are all in compliance with FDA 21 CFR 210, 211, 1271, FDA cGMP, and GTP standards; CA GMP Drug Manufacturing License Certified
- Fully equipped for GMP cell manufacturing and gene editing projects
- Supporting spaces: GMP material release room (~150 sf), process development space, QC/QA space (~5000 sf), and storage space (~300 sf)



