Newsletter
Neurotoxicity Screening
Cell-based in vitro assays for drug toxicity are becoming crucial tools to screen new drug candidates because they are inexpensive, efficient, and ethically compatible preliminary screening alternatives to the more expensive animal testing models.
Applied StemCell offers a one-stop shopping solution for neurological compound screening. Starting with the engineering of iPSCs from healthy and diseased patient samples; developing disease models via gene editing for drug target discovery and efficacy testing; differentiation into cell lineage(s); cell line model characterization; and ending with a comprehensive panel of cell-based tests for drug efficacy, neurotoxicity, and target discovery that are regulatory compliant.
Products and Services
Technical Details
Leverage ASC’s unique expertise in stem cell and genome editing technologies to forge ahead with your preclinical drug discovery pipeline. We offer comprehensive iPSC-based assay solutions for neurotoxicity testing or efficacy screening of your CNS drugs. You can use our ready-to-use iPSC-derived neuronal lineage cells (neurons and astrocytes) or we can start from your patient samples to reprogram iPSCs and differentiate them. Choose how you want your assays and we can customize the project to your specifications.
|
1. Generation of iPSCs lines Control iPSCs Engineered iPSCs to model disease Engineered reporter lines Patient-specific iPSC lines CRISPR Point Mutation Correction |
2. Differentiation to Neural Cells Neural stem cells (NSC) Neurons (dopaminergic, cortical) Astrocytes Oligodendrocytes Microglia |
3. Disease Modeling & Drug Screening Neurotoxicology assays Neuroprotection screening CNS drug efficacy testing Screening for new drug targets |
We provide stage-specific phenotype assays in our neurotoxicity platform:
- Dose-response curve for neurotoxicity and drug efficacy
- Screening in multiple cell types: iPSCs, NSCs, neurons and glial cells
- Cellular Morphology
- Synaptogenesis, network formation using neuronal co-cultures
|
Screening |
Types of Assays |
Estimated Timeline |
|
Cytotoxicity & Cell Viability Assays |
MTT/ MTS cell proliferation assay LDH, Necrosis and Apoptosis assays Luciferase (bioluminescence) expression cAMP level measurement |
4-6 weeks |
|
Mitochondrial Toxicity Testing |
Enzyme activity Volume fraction detection |
2-4 weeks |
|
Functional Assays |
Calcium influx/ imaging Electrophysiology: Multielectrode array (MEA) analysis and Patch clamp recording |
8-12 weeks |
|
Quantitative Gene Expression |
qPCR RNA-seq using NGS (next generation sequencing) |
2-8 weeks |
|
Morphology |
Neurite growth assay Biomarker screening |
2-4 weeks |
|
Custom Assays |
iPSC generation; characterization; gene editing; differentiation Custom assay development |
Based on project requirements |
We offer various neuronal lineage cells (Dopaminergic neurons, GABAergic/mixed neurons, microglia, astrocytes and oligodendrocytes, and neuronal co-cultures) or custom service to differentiate your patient samples/ iPSCs to neurons and glial cells for neurotoxicity testing that are derived from:
- Parental/ Control iPSCs derived from multiple donors and tissue samples (cord blood; PBMCs; fibroblasts)
- Patient-derived iPSC lines
- Neuronal disease-specific isogenic knockout lines (Parkinson’s disease, Alzheimer’s, autism)
- Reporter iPSC lines (neural lineage-specific reporter iPSCs, iPSCs with reporter inserted at a safe harbor locus)
Custom cells: We also generate iPSCs and genome edit iPSCs for specialized projects. Please inquire for details.
It is important to test a variety of cell types because no one-test can be used to examine all aspects of the human nervous system. Our cell-based assay for neurotoxicity testing is the perfect solution:
- Our testing is highly reproducible providing confidence in all data generated.
- The differentiated neuronal cells are fully characterized by immunocytochemistry and whole genome profiling.
- iPSC models can be patient-specific and can be differentiated into many neural cell types helping to fulfill the promise of personalized medicine.
- In vitro cell-based assays for neurotoxicity testing are now considered effective and regulatory compliant, by key governmental agencies, to assess risk.
- A cell-based toxicity testing assay allows for prioritization of decisions to be made to move the most promising compounds into appropriate animal studies quickly.
Let us do your preclinical neurotoxicity testing with our consistent and reliable source of neuronal cell types. Contact us to discuss the cell-based assays we have for testing toxicity and the endpoints that make sense for your drug discovery project.
Application Notes
Neurotoxicity Screening of Drug Candidates using iPSC-derived Neuronal Lineage Cells
Using iPSC-differentiated Neural Lineage Cells for Determining Cell Viability in Neurotoxicity Assays
A.
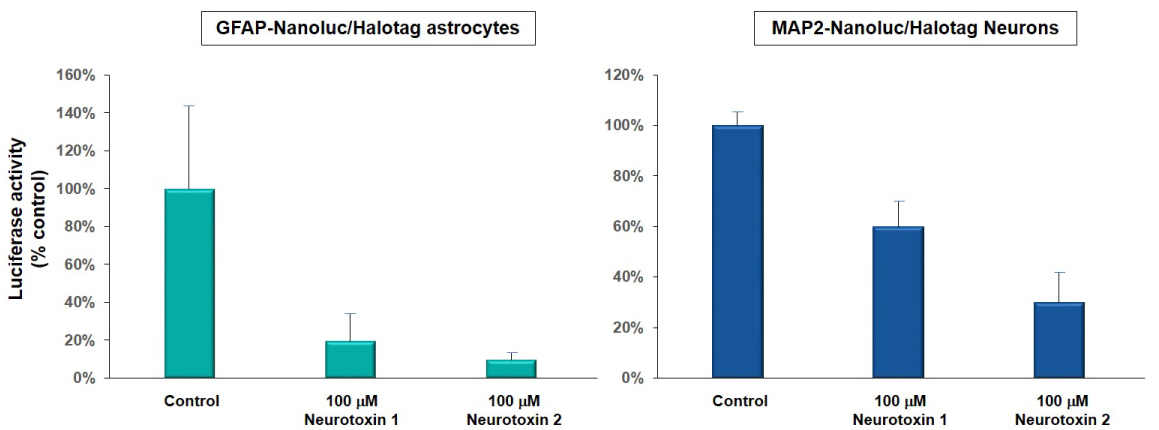
Figure 1A. Luciferase activity (bioluminescence) was used to detect the cell viability of astrocytes and neurons derived from neural reporter iPSC lines (GFAP-Nanoluc/Halotag, ASE-9501; green and MAP2/Nanoluc-Halotag, ASE-9500; blue) when exposed to 100 µM of neurotoxin 1 and 2. Neurotoxin 1 reduced luciferase activity in astrocytes by ~ 80% and in neurons by ~40%. Neurotoxin 2 reduced luciferase activity in astrocytes by ~90% and in neurons by ~70%. Luciferase activity was measured (as % of control; DMSO-treated cells) to determine the extent of cytotoxicity of the compounds. These results were similar to toxicity measured by the MTT assay.
B.
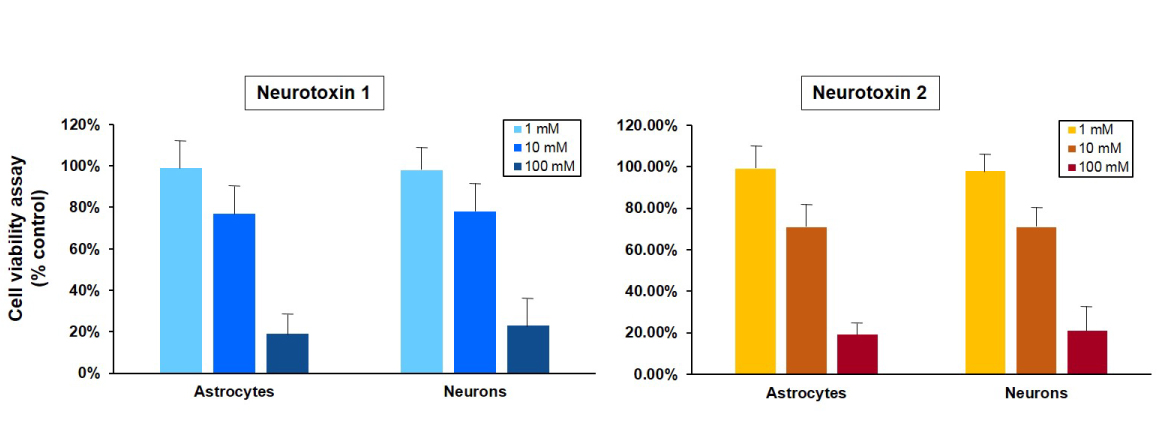
Figure 1B. Cell viability of astrocytes and neurons after exposure to neurotoxins. Astrocytes and neurons derived from a control iPSC line (ASE-9109) were exposed to concentrations of 1, 10 and 100 μM concentrations of two neurotoxins, 1 and 2. Ce ll viability was evaluated using MTT assay (MTT tetrazolium salt) and cell survival was expressed as % of absorbance of viable cells normalized to control (DMSO-treated cells). The 1 µM concentration of both neurotoxins was not significantly cytotoxic in both cell types while there was mild cytotoxicity observed at the 10µM concentrations of the neurotoxins. The 100 µM concentration of both neurotoxins was significantly cytotoxic and resulted in ~80% reduction in cell viability in astrocytes and neurons. The results of this assay were similar to the expression of luciferase in astrocytes and neurons derived from a neuronal-reporter iPSC line (Figure 1A.).
iPSC-differentiated Neurons Can be Used for Screening Potential Neuroprotective Compounds
iPSCs and derived neurons can be used for testing neuroprotective effects of compounds using MTT assay.
Table 1. Drugs that were neuroprotective in iPSC and derived neuronal cells, and used for human clinical trials
|
Neurotransmitter/ MAO Inhibitors: |
Rasagiline, selegiline, nicotine, topiramate, amantadine, zonisamide, taurine |
|
Antioxidant/ Mitochondrial Stabilizers: |
Resveratrol, N-acetyl cysteine, lipoic acid, epigallocatechin gallate, creatine |
|
Anti-inflammatories: |
Rolipram, indomethacin, 7-nitroindazole, 3-aminobenzamide, phenanthridone |
Table 2. Drug that were not neuroprotective in iPSC-based models but were neuroprotective in conventional cell lines and animal models:
|
Neurotransmitter/ MAO Inhibitors: |
Donepezil, caffeine, theophylline, pergolide, apomorphine, riluzole, pramipexole |
|
Antioxidant/ Mitochondrial Stabilizers: |
Ascorbic acid, coenzyme Q10, uric acid, folic acid, ropinirole |
|
Anti-inflammatories: |
Minocycline, estradiol, clioquinol, plicamycin |
Dopaminergic (DA) neurons derived from control iPSC lines were used to screen neurological compounds that have been shown to be neuroprotective in rodent and cell line models (Table 1 and 2). These derived-primary DA neurons were grown in 96-well plates and pretreated with one of each of the selected compounds. The cells were then exposed to either rotenone or MPP+ that are commonly used in dopamine toxicity models and cell viability was determined using the MTT assay. Only 18 out of the compounds (Table 1) were found to be neuroprotective in these iPSC-derived DA neurons, and these 18 compounds have been used in human Parkinson's disease clinical trials to test for neuroprotection. The rest of the compounds (Table 2) have either not been used in clinical trials or were not neuroprotective. This highlights the importance of using iPSC-derived neurons for large-scale screening neurological drugs before evaluating their efficacy in animal and human studies.