Cardiomyocyte Differentiation Service Details
iPSC-derived cardiomyocytes are heart muscle cells that are generated from induced pluripotent stem cells (iPSCs), which are reprogrammed adult cells that have the ability to differentiate into many different cell types, including cardiomyocytes. Cardiomyocytes are the cells responsible for the contraction of the heart and the pumping of blood throughout the body. The generation of iPSC-derived cardiomyocytes involves a process of differentiation that mimics the natural development of heart muscle cells in the developing embryo. This process involves the sequential activation of different genes and signaling pathways that direct the iPSCs towards a cardiomyocyte fate.
iPSC-derived cardiomyocytes have several important applications in research and medicine. For example, they can be used as a model for studying heart diseases and drug toxicity. By generating cardiomyocytes from iPSCs derived from patients with heart diseases, researchers can study the disease mechanisms and test potential therapies in a dish.
In addition, iPSC-derived cardiomyocytes have the potential to be used in regenerative medicine. For patients with heart damage or heart failure, transplantation of healthy cardiomyocytes could be a potential treatment option. However, donor hearts are scarce, and transplantation can be associated with complications such as rejection and infection. iPSC-derived cardiomyocytes could offer a potential alternative, providing a source of functional heart muscle cells that can be generated in large quantities and tailored to individual patient needs.
Overall, the generation of iPSC-derived cardiomyocytes has the potential to advance our understanding of heart diseases and lead to new therapies for patients with heart conditions.
Standard Packaging:
- Ship 1-2 million iPS cells (1-2 cryovials; 1 million cells/vial); OR
- With pathogen and SARS-CoV-2 test results
- Optional Pathogen Testing Service is available
- With pathogen and SARS-CoV-2 test results
- Select one of our Control iPS Cell Lines; OR
- Let ASC generate your iPSCs from your human or non-human samples
Standard Deliverables:
- Cardiomyocytes differentiated from iPSCs; options available for quantity (Ex. small scale:2 million differentiated cells; medium scale: 2-10 million differentiated cells; large scale: 10-100 million cells).
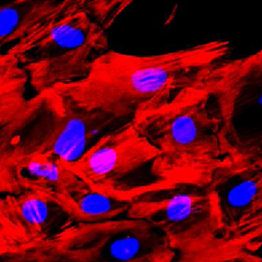
- QC data: Antibody staining (cTnT, alpha-SMA) and video of beating differentiated cells (before cryopreservation), recovery, and mycoplasma tests.
- Milestone updates/ reports
Standard Workflow and Timeline:
| Service |
| iPSC Recovery and Expansion |
| Pathogen Screening (Optional Add-On) |
| Directed differentiation to cardiomyocyte |
| QC: Antibody staining (cTnT, SMA); video (beating cells); recovery test |
Timeline: 2 weeks
Applications:
- Disease Modeling
- In vitro drug testing and cardiotoxicity screening
- Transplantation research
- Study approaches to regenerative medicine
Video of Beating Cardiomyocytes Differentiated from a Control iPSC Line
The videos seen on the top of the page show beating, cardiomyocytes that were differentiated from an integration-free, control human iPSC line. The cardiomyocytes were on day 3 of recovery after cryopreservation (cryopreserved on day 10 of post-differentiation). The videos were taken by bright-field microscopy at 20x magnification.