Newsletter
GMP Grade iPSC Services & Products
Leading iPSC CDMO, Ready-to-use iPSC GMP Master Cell Bank & GMP Cell Therapy Services
ASC received its Drug Manufacturing License from the California Food and Drug Branch in 2021. Since then, iPSC processes have been established for cell banking and iPSC reprogramming, genome editing (CRISPR and TARGATT™), and differentiation that are compliant with FDA (US) and EMA (EU) regulations. As a CRO/CDMO committed to providing high-quality services and products, we follow quality control protocols based on ICH-Q5A/D guidelines for the development of safe iPSC and iPSC-derived products in our ISO Class 5 and 7 (Class 10,000 and 100) clean room.
In addition to our cGMP-compliant iPSC generation service, we offer well-characterized, ready-to-use GMP-grade iPSCs (Cord Blood CD34+, Male) and cGMP-Compliant TARGATTTM Master iPSCs that can be engineered to express almost any gene of interest (e.g., CAR genes). The genome-edited cells can be further differentiated into various cell types, including NK cells, T cells neurons, RPE cells, Cardiomyocytes, Neurons, and more. Our team of experts can even work with customer-provided cell lines. We currently offer:
- GMP iPSC Master Cells
- iPSC GMP Gene Editing Service
- iPSC GMP Differentiation includes NPCs, HPCs, CAR-NK, RPEs
- iPSC GMP Cell Manufacturing/Cell Banking
Current Good Manufacturing Practice (cGMP):
Regulations ASC complies with as a cell processing GMP facility <Located in CA, USA>
- 21 CFR Part 210
- Current Good Manufacturing Practice in Manufacturing, Processing, Packing or Holding of Drugs; General
- 21 CFR Part 211
- Current Good Manufacturing Practice for Finished Pharmaceuticals
- 21 CFR Part 1271
- Human Cells, Tissues, and Cellular and Tissue-Based Products
- Current Good Tissue Practice
1. GMP Grade iPSCs
- cGMP-Grade iPSC Line: The cells are well characterized and can be genetically modified and further differentiated to various cell types.
- Source cell type: CD34+ Cord Blood, male
- Reprogramming method: Episomal
- Master cell bank or working cell bank available
- Drug master file with US FDA
- Matching Research iPSC Line is Available
Product QC:
- Sterility/mycoplasma
- Identity: SNP Trace
- Pluripotency: image, AP stain, Oct4/TRA-1-60 stain, differentiation in vitro to 3 germ layers
- Genetic stability: karyotyping by G-banding
- Viability
- cGMP-Compliant TARGATTTM Master iPSCs: Ready to insert your GOI. High-quality iPSCs with a pre-engineered landing pad for site-specific, unidirectional gene insertion at a safe harbor locus mediated by TARGATTTM
-
- Engineered from ASC’s GMP grade iPSCs using TARGATTTM (No CRISPR)
- GMP SOP
- All reagents are CGT grade (animal, serum-free)
- Documentation and QC/QA follow GMP SOP
- Certificate of Analysis provided upon request
- All testing follows GMP requirements
- License-ready
- Engineered from ASC’s GMP grade iPSCs using TARGATTTM (No CRISPR)
2. GMP iPSC Services
- cGMP iPSC Expansion & Banking; Master Cell Bank (MCB); Working Cell Bank (WCB)
- Cell Banking & Storage with 24/7 Monitoring System
- Customized iPSC Generation (Episomal Method)
- iPSC Gene Editing: TARGATT™ Gene Editing
- iPSC Gene Editing: CRISPR/Cas9 Gene Editing
- In-Process QC (Reagent QC (plasmid map/sequence), Validation (cell, gRNA, integrase), and more)
- iPSC Differentiation: Customer Provide Protocol
- iPSC Differentiation: ASC’s SOP
- TARGATTTM Master iPSC Platform for Allogeneic Products
- iPSC-based Assay Services (In Vitro and In Vivo)
iPSC GMP SERVICES: iPSC Generation, Expansion, and Banking
|
cGMP iPSC Expansion and Banking |
|||
|
|
Number of Vials |
Cell Count |
Other Deliverables |
|
Master Cell Bank |
50-200 vials |
1x10^6 cells/vial |
QC and CoA |
|
Working Cell Bank |
50-200 vials |
1x10^6 cells/vial |
QC and CoA |
*Stocking of Frozen Cells (Annual Storage): 24/7 monitoring
|
Customized iPSC Generation (Episomal Method) |
||||
|
|
Number of Clones |
Number of Vials |
Cell Count |
Other Deliverables |
|
Seed Bank |
3 Clones |
10 vials |
1x10^6 cells/vial |
QC Characterization and CoA |
GMP SERVICES: iPSC Gene Editing
With 13+ years of stem cell & genome editing experience, ASC offers high-quality gene editing services using the latest technology, CRISPR and TARGATTTM. For all CRISPR, fee-for service gene editing projects, the customer must obtain a CRISPR license. With ASC’s TARGATTTM technology, customers can avoid having to obtain additional licensing and only need a single license from ASC!
|
iPSC Gene Editing |
|
|
Service |
Details |
|
CRISPR/Cas9 Gene Editing |
|
|
TARGATT™ Gene Editing |
|
|
GMP-like Plasmid Production For Gene Editing |
|
In-Process QC:
- Reagent QC (plasmid map/sequence)
- Validation (cell, gRNA, integrase)
- Transfection pool genotyping
- Clone genotyping
TARGATTTM Site-Specific Knockin Technology
Unlike other gene editing companies, ASC has a unique gene-editing technology, TARGATTTM, that enables site-specific, irreversible gene insertion at a safe-harbor locus, H11 or ASC2. The unidirectional gene insertion at an active, intergenic locus does not disrupt other internal genes. TARGATTTM works well in non-dividing cells and permits single-copy insertion of large fragments up to 20kb. ASC has observed high integration efficiencies and medium to high protein expression in stable cells.
The NIH awarded Applied StemCell with a $2M SBIR grant for the improvement of its hTARGATTTM technology for human gene and cell therapy. According to the NIH ASC’s proprietary gene editing technology
“could efficiently and safely treat more than ten thousand monogenic human diseases.”
- NIH grant review group summary statement
GMP SERVICES: iPSC DIFFERENTIATION
Integration-free protocols to differentiate iPSCs to various committed somatic lineages, including NK cells, T cells, RPE cells, neurons, and many others.
|
iPSC Differentiation |
|
|
Service |
Details |
|
iPSC Differentiation: Customer provides protocol |
|
|
iPSC Differentiation: ASC’s SOP |
|
GMP SERVICES: iPSC DIFFERENTIATION TO COMMITED SOMATIC LINEAGES
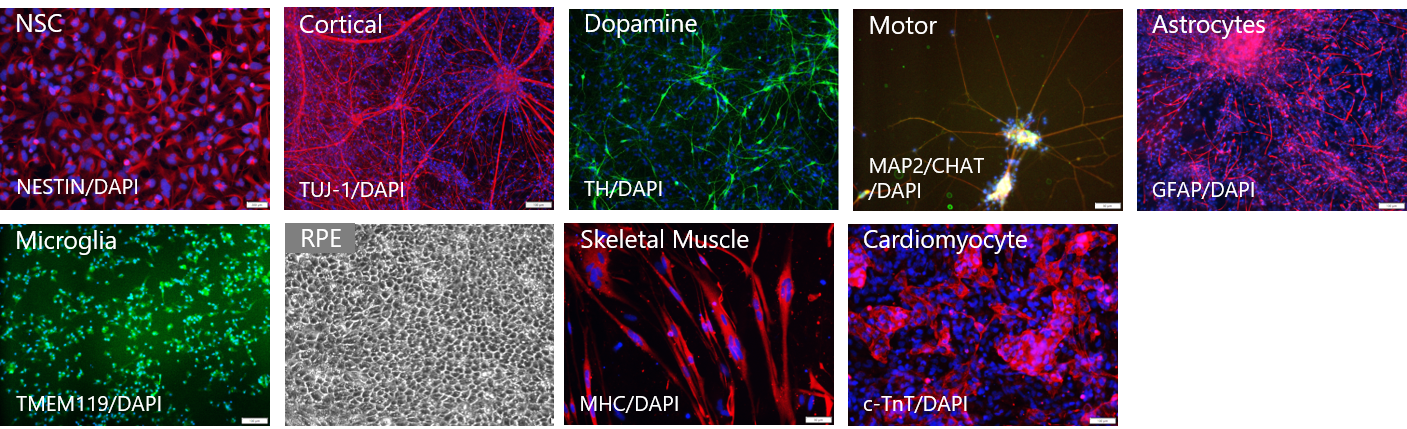
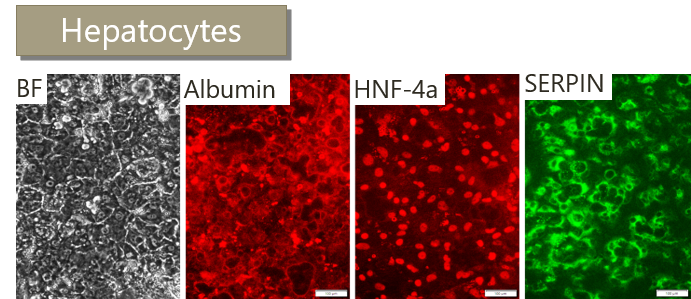
GMP SERVICES: iPSC DIFFERENTIATION TO NK CELLS (iNK)
Characterization of the ASE-9708 NK Cells
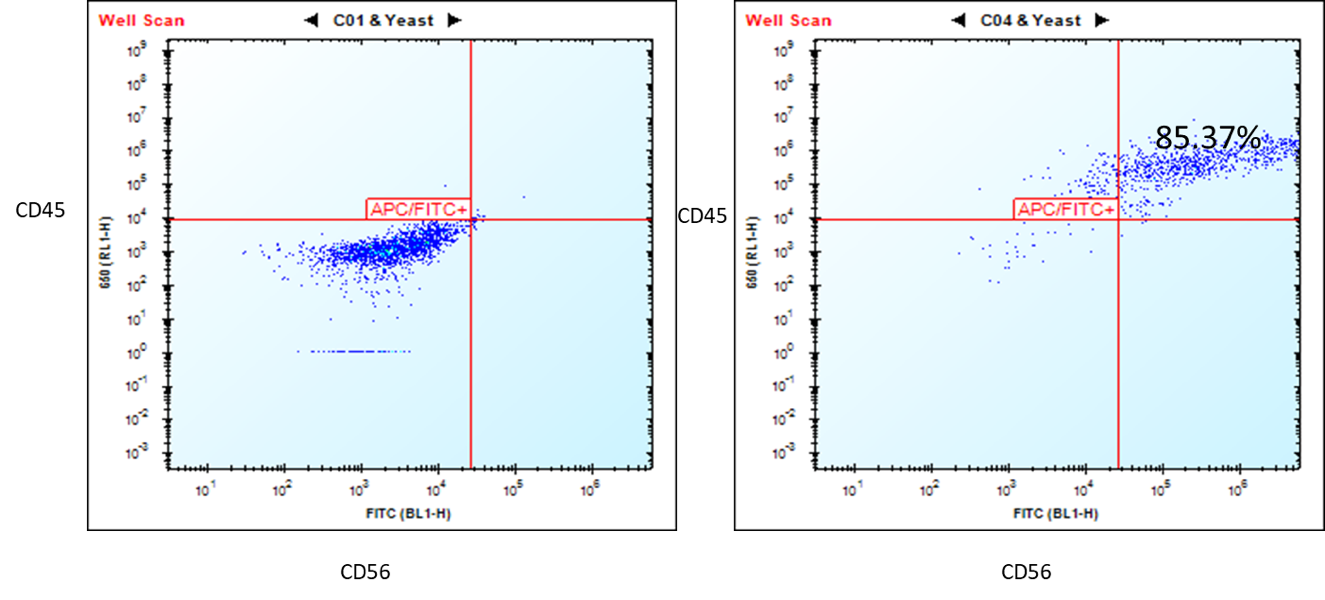
Figure 1. Flow cytometry analysis of ASE-9708 iPSC-derived NK cells for NK cell biomarkers. Cryopreserved NKs, differentiated from Applied StemCell’s control iPSC line, ASE-9211 were recovered in NK culture media. The cells were stained with NK cell markers, CD45 and CD56 at day 2. Left: Isotype control antibodies. Right: CD45/CD56.
GMP SERVICES: iPSC DIFFERENTIATION TO RPE CELLS
Characterization of the ASE-9710 RPE Cells
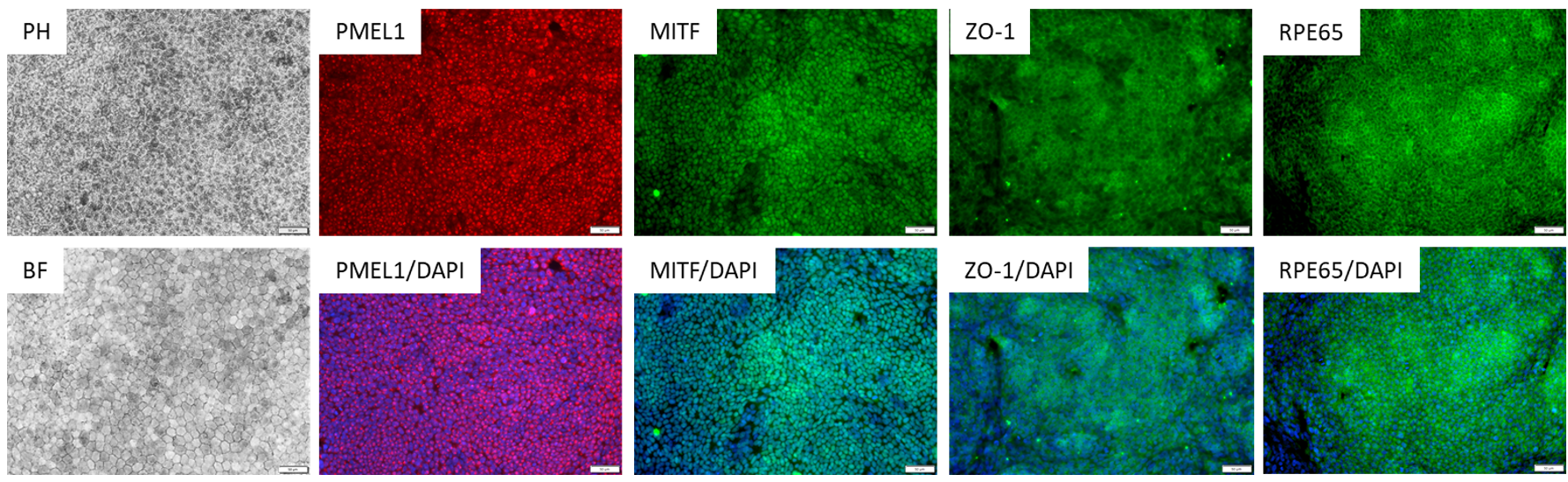
Figure 2. Immunostaining of ASE-9710 iPSC-derived Retinal Pigment Epithelium Cells for retinal pigment
epithelium biomarkers. Cryopreserved RPE cells, differentiated from Applied StemCell’s control iPSC line, ASE9211 were recovered in RPE culture media. The cells were stained with RPE markers, PMEL1, MITF, ZO-1 and
RPE65.
TARGATTTM Master iPSC Platform for Allogeneic Products
The comprehensive TARGATTTM Master iPSC platform permits the safe and efficient insertion of any gene of interest at a safe harbor locus in the TARGATTTM Master iPSCs. This process is facilitated by TARGATTTM integrase, and the engineered cells can be further differentiated to various cells types.
- TARGATTTM Master iPSCs
Pluripotent stem cells with a pre-engineered landing pad in a safe genomic locus for repeated, fast, and efficient gene knock-in by TARGATTTM integrase
- Engineered from ASC’s GMP grade iPSC using TARGATT™ technology only (no CRISPR)
- All reagents are CGT grade (animal, serum-free)
- Follow GMP SOPs
- Documentation and QC/QA follow GMP SOP
- Certificate of Analysis provided
- All testing follows GMP requirement
- iPSC Differentiation
For the creation of unlimited sources of off-the-shelf allogenic therapeutic cells.
Products and Services
Technical Details
GMP Facility
ASC's GMP facility is set for iPSC generation, expansion, gene editing, and differentiation as well as general cell banking processes. ASC’s GMP facility includes manufacturing space, the storage for raw and finished products, and support lab areas. ASC’s GMP facility operates under the guidelines established by the CFR (Code of Federal Regulations) Title 21, Parts 1271 (Current Good Manufacturing for Human Cells, Tissues, and Cellular and Tissue-Based Products).
Application Notes
Applications in Cell Therapy
The off-the-shelf master iPSCs have the potential to revolutionize immunotherapy. The engineered human induced pluripotent stem cells with a landing pad at a genomic safe-harbor locus can be used to insert a therapeutic gene of interest to generate an iPSCs that carry a single copy of the gene needed for therapeutic studies.
Universal Modifiable iPSC in CAR-NK Therapy
Since ASC’s Master iPSC line holds a TARGATTTM site at a safe-harbor locus, a single copy of a gene of interest such as CAR can be inserted into the genome. ASC could generate an iPSC line with a specific CAR, and use its integration-free method to differentiate the iPSCs with CAR to a committed cell type such as natural killer (NK) cells. With the rise in CAR-NK and CAR-T therapies, ASC’s TARGATTTM and iPSC differentiation capabilities offer an alternative, effective method for the development of novel CAR-NK cell therapies.
Support Materials



